Antibacterial and Fluorescence Staining Properties of an Innovative GTR Membrane Containing 45S5BGs and AIE Molecules In Vitro
- PMID: 35214970
- PMCID: PMC8874606
- DOI: 10.3390/nano12040641
Antibacterial and Fluorescence Staining Properties of an Innovative GTR Membrane Containing 45S5BGs and AIE Molecules In Vitro
Abstract
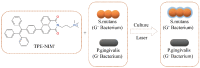
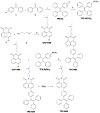
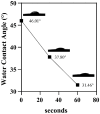
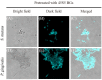
This study aimed to add two functional components-antibacterial 45S5BGs particles and AIE nanoparticles (TPE-NIM+) with bioprobe characteristics-to the guided tissue regeneration (GTR) membrane, to optimize the performance. The PLGA/BG/TPE-NIM+ membrane was synthesized. The static water contact angle, morphologies, and surface element analysis of the membrane were then characterized. In vitro biocompatibility was tested with MC3T3-E1 cells using CCK-8 assay, and antibacterial property was evaluated with Streptococcus mutans and Porphyromonas gingivalis by the LIVE/DEAD bacterial staining and dilution plating procedure. The fluorescence staining of bacteria was observed by Laser Scanning Confocal Microscope. The results showed that the average water contact angle was 46°. In the cytotoxicity test, except for the positive control group, there was no significant difference among the groups (p > 0.05). The antibacterial effect in the PLGA/BG/TPE-NIM+ group was significantly (p < 0.01), while the sterilization rate was 99.99%, better than that in the PLGA/BG group (98.62%) (p < 0.01). Confocal images showed that the membrane efficiently distinguished G+ bacteria from G- bacteria. This study demonstrated that the PLGA/BG/TPE-NIM+ membrane showed good biocompatibility, efficient sterilization performance, and surface mineralization ability and could be used to detect pathogens in a simple, fast, and wash-free protocol.
Keywords: aggregation-induced emission nanoparticles; antibacterial; antibacterial differentiation; bioactive glass; bioprobe; guided tissue regeneration (GTR).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

