Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode
- PMID: 35214973
- PMCID: PMC8877794
- DOI: 10.3390/nano12040645
Electrochemical Detection of Ascorbic Acid in Oranges at MWCNT-AONP Nanocomposite Fabricated Electrode
Abstract
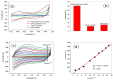
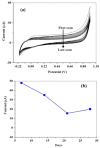
Ascorbic acid (AA) is an essential vitamin in the body, influencing collagen formation, as well as norepinephrine, folic acids, tryptophan, tyrosine, lysine, and neuronal hormone metabolism. This work reports on electrochemical detection of ascorbic acid (AA) in oranges using screen-print carbon electrodes (SPCEs) fabricated with multi-walled carbon nanotube- antimony oxide nanoparticle (MWCNT-AONP) nanocomposite. The nanocomposite-modified electrode displayed enhanced electron transfer and a better electrocatalytic reaction towards AA compared to other fabricated electrodes. The current response at the nanocomposite-modified electrode was four times bigger than the bare electrode. The sensitivity and limit of detection (LOD) at the nanocomposite modified electrode was 0.3663 [AA]/µM and 140 nM, respectively, with linearity from 0.16-0.640 μM and regression value R2 = 0.985, using square wave voltammetry (SWV) for AA detection. Two well-separated oxidation peaks were observed in a mixed system containing AA and serotonin (5-HT); and the sensitivity and LOD were 0.0224 [AA]/µA, and 5.85 µΜ, respectively, with a concentration range from 23 to 100 µM (R2 = 0.9969) for AA detection. The proposed sensor outperformed other AA sensors reported in the literature. The fabricated electrode showed great applicability with excellent recoveries ranging from 99 to 107 %, with a mean relative standard deviation (RSD) value of 3.52 % (n = 3) towards detecting AA in fresh oranges.
Keywords: antimony oxide nanoparticles; ascorbic acid; electrochemical sensors; nanocomposite; oranges.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Fadhel D.H. Spectrophotometric determination of ascorbic acid in aqueous solutions. Al-Nahrain J. Sci. 2012;15:88–94. doi: 10.22401/JNUS.15.3.13. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

