Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins
- PMID: 35214974
- PMCID: PMC8877803
- DOI: 10.3390/nano12040644
Minimizing the Silver Free Ion Content in Starch Coated Silver Nanoparticle Suspensions with Exchange Cationic Resins
Abstract
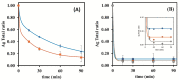
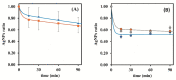
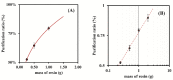
This work describes the optimization of a methodology for the reduction of silver ions from silver nanoparticle suspensions obtained from low-yield laboratory procedures. The laboratory synthesis of silver nanoparticles following a bottom-up approach starting from silver nitrate, originates silver ions that were not reduced to their fundamental state for nanoparticles creation at the end of the process. However, it is well known that silver ions can easily influence chemical assays due to their chemical reactivity properties and can limit biological assays since they interfere with several biological processes, namely intracellular ones, leading to the death of living cells or organisms. As such, the presence of silver ions is highly undesirable when conducting biological assays to evaluate the influence of silver nanoparticles. We report the development of an easy, low-cost, and rapid methodology that is based on cation exchange resins to minimize the silver ion content in a raw suspension of silver nanoparticles while preserving the integrity of the nanomaterials. This procedure preserves the physical-chemical properties of the nanoparticles, thus allowing the purified nanoparticulate systems to be biologically tested. Different types of cationic resins were tested, and the developed methodology was optimized by changing several parameters. A reduction from 92% to 10% of free silver/total silver ratio was achieved when using the Bio-Rad 50W-X8 100-200 mesh resin and a contact time of 15 min. Filtration by vacuum was used to separate the used resin from the nanoparticles suspension, allowing it to be further reused, as well as the purified AgNPs suspension.
Keywords: AgNPs; cation exchange; green chemistry; purification; resin; separation; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Liu J.-F., Yu S.-J., Yin Y.-G., Chao J.-B. Methods for separation, identification, characterization and quantification of silver nanoparticles. TrAC Trends Anal. Chem. 2012;33:95–106. doi: 10.1016/j.trac.2011.10.010. - DOI
-
- Lee S.H., Salunke B.K., Kim B.S. Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using Magnolia kobus plant leaf extracts. Biotechnol. Bioprocess Eng. 2014;19:169–174. doi: 10.1007/s12257-013-0561-4. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

