Affibody Modified G-quadruplex DNA Micelles Incorporating Polymeric 5-Fluorodeoxyuridine for Targeted Delivery of Curcumin to Enhance Synergetic Therapy of HER2 Positive Gastric Cancer
- PMID: 35215023
- PMCID: PMC8879187
- DOI: 10.3390/nano12040696
Affibody Modified G-quadruplex DNA Micelles Incorporating Polymeric 5-Fluorodeoxyuridine for Targeted Delivery of Curcumin to Enhance Synergetic Therapy of HER2 Positive Gastric Cancer
Abstract
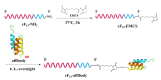
Combination chemotherapy is emerging as an important strategy for cancer treatment with decreased side effects. However, chemotherapeutic drugs with different solubility are not easy to realize co-delivery in traditional nanocarriers. Herein, an affibody modified G-quadruplex DNA micellar prodrug (affi-F/GQs) of hydrophilic 5-fluorodeoxyuridine (FUdR) by integrating polymeric FUdRs into DNA strands is developed for the first time. To achieve synergistic efficacy with hydrophobic drugs, curcumin (Cur) is co-loaded into affi-F/GQs micelles to prepare the dual drug-loaded DNA micelles (Cur@affi-F/GQs), in which affibody is employed as a targeting moiety to facilitate HER2 receptor-mediated uptake. Cur@affi-F/GQs have a small size of approximately 130 nm and exhibit excellent stability. The system co-delivers FUdR and Cur in a ratiometric manner, and the drug loading rates are 21.1% and 5.6%, respectively. Compared with the physical combination of FUdR and Cur, Cur@affi-F/GQs show higher cytotoxicity and greater synergistic effect on HER2 positive gastric cancer N87 cells. Surprisingly, Cur@affi-F/GQs significantly enhance the expression and activity of apoptosis-associated proteins in Bcl-2/Bax-caspase 8, 9-caspase 3 apoptotic pathway, which is the main factor in the death of tumor cells induced by FUdR. Overall, this nanoencapsulation is a promising candidate for the targeted co-delivery of drugs with significant differences in solubility.
Keywords: 5-fluorodeoxyuridine; DNA micelles; G-quadruplex; affibody; curcumin; synergetic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
















References
-
- Ren Y., Wang R., Gao L., Li K., Zhou X., Guo H., Liu C., Han D., Tian J., Ye Q., et al. Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. J. Controlled. Release. 2016;228:74–86. doi: 10.1016/j.jconrel.2016.03.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

