The Coxsackievirus and Adenovirus Receptor Has a Short Half-Life in Epithelial Cells
- PMID: 35215116
- PMCID: PMC8880067
- DOI: 10.3390/pathogens11020173
The Coxsackievirus and Adenovirus Receptor Has a Short Half-Life in Epithelial Cells
Abstract
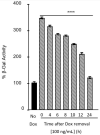
The coxsackievirus and adenovirus receptor (CAR) is an essential cellular protein that is involved in cell adhesion, cell signaling, and viral infection. The 8-exon encoded isoform (CAREx8) resides at the apical surface of polarized epithelia, where it is accessible as a receptor for adenovirus entering the airway lumen. Given its pivotal role in viral infection, it is a target for antiviral strategies. To understand the regulation of CAREx8 and determine the feasibility of receptor downregulation, the half-life of total and apical localized CAREx8 was determined and correlated with adenovirus transduction. Total and apical CAREx8 has a relatively short half-life of approximately 2 h. The half-life of apical CAREx8 correlates well with adenovirus transduction. These results suggest that antiviral strategies that aim to degrade the primary receptor for apical adenovirus infection will be effective within a relatively short time frame after application.
Keywords: coxsackievirus and adenovirus receptor; half-life; human adenovirus; polarized epithelia.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- William S.M., Wold M.S.H. Adenoviruses. In: David M., Knipe P.M.H., editors. Fields Virology. Volume 2. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2007. pp. 2395–2436.
Grants and funding
LinkOut - more resources
Full Text Sources

