Non-Canonical Host Intracellular Niche Links to New Antimicrobial Resistance Mechanism
- PMID: 35215166
- PMCID: PMC8876822
- DOI: 10.3390/pathogens11020220
Non-Canonical Host Intracellular Niche Links to New Antimicrobial Resistance Mechanism
Abstract
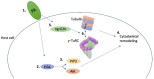
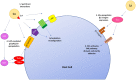
Globally, infectious diseases are one of the leading causes of death among people of all ages. The development of antimicrobials to treat infectious diseases has been one of the most significant advances in medical history. Alarmingly, antimicrobial resistance is a widespread phenomenon that will, without intervention, make currently treatable infections once again deadly. In an era of widespread antimicrobial resistance, there is a constant and pressing need to develop new antibacterial drugs. Unraveling the underlying resistance mechanisms is critical to fight this crisis. In this review, we summarize some emerging evidence of the non-canonical intracellular life cycle of two priority antimicrobial-resistant bacterial pathogens: Pseudomonas aeruginosa and Staphylococcus aureus. The bacterial factors that modulate this unique intracellular niche and its implications in contributing to resistance are discussed. We then briefly discuss some recent research that focused on the promises of boosting host immunity as a combination therapy with antimicrobials to eradicate these two particular pathogens. Finally, we summarize the importance of various strategies, including surveillance and vaccines, in mitigating the impacts of antimicrobial resistance in general.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; antibiotic resistance; non-canonical intracellular pathogen.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021. World Health Organization; Geneva, Switzerland: 2021. Licence: CC BY-NC-SA 3.0 IGO.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

