Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms
- PMID: 35215210
- PMCID: PMC8879499
- DOI: 10.3390/pathogens11020268
Metabolic Alteration of Trypanosoma cruzi during Differentiation of Epimastigote to Trypomastigote Forms
Abstract
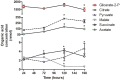
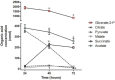
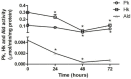
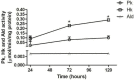
Intracellular parasites such as Trypanosoma cruzi need to acquire valuable carbon sources from the host cell to replicate. Here, we investigated the energetic metabolism of T. cruzi during metacyclogenesis through the determination of enzymatic activities and quantification by HPLC of glycolytic and Krebs cycle short-chain carboxylic acids. Altered concentrations in pyruvate, acetate, succinate, and glycerate were measured during the growth of epimastigote in the complex medium BHI and their differentiation to trypomastigotes in the chemically defined medium, TAU3AAG. These alterations should represent significant differential metabolic modifications utilized by either form to generate energy. This paper is the first work dealing with the intracellular organic acid concentration measurement in T. cruzi parasites. Although it confirms the previous assumption of the importance of carbohydrate metabolism, it yields an essential improvement in T. cruzi metabolism knowledge.
Keywords: Trypanosoma cruzi; carbohydrate metabolism; carboxylic acid; enzyme activity; high-performance liquid chromatography; organic acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
In vitro effects of citral on trypanosoma cruzi metacyclogenesis.Mem Inst Oswaldo Cruz. 2010 Dec;105(8):1026-32. doi: 10.1590/s0074-02762010000800012. Mem Inst Oswaldo Cruz. 2010. PMID: 21225200
-
Differentiation of Trypanosoma cruzi epimastigotes: metacyclogenesis and adhesion to substrate are triggered by nutritional stress.J Parasitol. 2000 Dec;86(6):1213-8. doi: 10.1645/0022-3395(2000)086[1213:DOTCEM]2.0.CO;2. J Parasitol. 2000. PMID: 11191893
-
Further in vivo evidence implying DNA apurinic/apyrimidinic endonuclease activity in Trypanosoma cruzi oxidative stress survival.J Cell Biochem. 2019 Oct;120(10):16733-16740. doi: 10.1002/jcb.28931. Epub 2019 May 17. J Cell Biochem. 2019. PMID: 31099049
-
May the epimastigote form of Trypanosoma cruzi be infective?Acta Trop. 2020 Dec;212:105688. doi: 10.1016/j.actatropica.2020.105688. Epub 2020 Sep 2. Acta Trop. 2020. PMID: 32888934 Review.
-
Amino acid metabolic routes in Trypanosoma cruzi: possible therapeutic targets against Chagas' disease.Curr Drug Targets Infect Disord. 2005 Mar;5(1):53-64. doi: 10.2174/1568005053174636. Curr Drug Targets Infect Disord. 2005. PMID: 15777198 Review.
Cited by
-
Regulatory Functions of Hypoxia in Host-Parasite Interactions: A Focus on Enteric, Tissue, and Blood Protozoa.Microorganisms. 2023 Jun 16;11(6):1598. doi: 10.3390/microorganisms11061598. Microorganisms. 2023. PMID: 37375100 Free PMC article. Review.
-
Hypoxia Effects on Trypanosoma cruzi Epimastigotes Proliferation, Differentiation, and Energy Metabolism.Pathogens. 2022 Aug 9;11(8):897. doi: 10.3390/pathogens11080897. Pathogens. 2022. PMID: 36015018 Free PMC article.
-
Interaction of Trypanosoma cruzi, Triatomines and the Microbiota of the Vectors-A Review.Microorganisms. 2024 Apr 25;12(5):855. doi: 10.3390/microorganisms12050855. Microorganisms. 2024. PMID: 38792688 Free PMC article. Review.
-
Cultivation of Protozoa Parasites In Vitro: Growth Potential in Conventional Culture Media versus RPMI-PY Medium.Vet Sci. 2023 Mar 27;10(4):252. doi: 10.3390/vetsci10040252. Vet Sci. 2023. PMID: 37104407 Free PMC article.
References
-
- Parodi-Talice A., Monteiro-Goes V., Arrambide N., Avila A.R., Duran R., Correa A., Dallagiovanna B., Cayota A., Krieger M., Goldenberg S., et al. Proteomic analysis of metacyclic trypomastigotes undergoing Trypanosoma cruzi metacyclogenesis. J. Mass Spectrom. 2007;42:1422–1432. doi: 10.1002/jms.1267. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

