Repurposing Antifungals for Host-Directed Antiviral Therapy?
- PMID: 35215323
- PMCID: PMC8878022
- DOI: 10.3390/ph15020212
Repurposing Antifungals for Host-Directed Antiviral Therapy?
Abstract
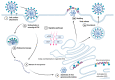
Because of their epidemic and pandemic potential, emerging viruses are a major threat to global healthcare systems. While vaccination is in general a straightforward approach to prevent viral infections, immunization can also cause escape mutants that hide from immune cell and antibody detection. Thus, other approaches than immunization are critical for the management and control of viral infections. Viruses are prone to mutations leading to the rapid emergence of resistant strains upon treatment with direct antivirals. In contrast to the direct interference with pathogen components, host-directed therapies aim to target host factors that are essential for the pathogenic replication cycle or to improve the host defense mechanisms, thus circumventing resistance. These relatively new approaches are often based on the repurposing of drugs which are already licensed for the treatment of other unrelated diseases. Here, we summarize what is known about the mechanisms and modes of action for a potential use of antifungals as repurposed host-directed anti-infectives for the therapeutic intervention to control viral infections.
Keywords: antifungals; azoles; drug repurposing; echinocandins; host-directed drug therapy; polyenes; viral infections.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Target Discovery for Host-Directed Antiviral Therapies: Application of Proteomics Approaches.mSystems. 2021 Oct 26;6(5):e0038821. doi: 10.1128/mSystems.00388-21. Epub 2021 Sep 14. mSystems. 2021. PMID: 34519533 Free PMC article.
-
Drug Repurposing in Medical Mycology: Identification of Compounds as Potential Antifungals to Overcome the Emergence of Multidrug-Resistant Fungi.Pharmaceuticals (Basel). 2021 May 20;14(5):488. doi: 10.3390/ph14050488. Pharmaceuticals (Basel). 2021. PMID: 34065420 Free PMC article. Review.
-
Strategy, Progress, and Challenges of Drug Repurposing for Efficient Antiviral Discovery.Front Pharmacol. 2021 May 4;12:660710. doi: 10.3389/fphar.2021.660710. eCollection 2021. Front Pharmacol. 2021. PMID: 34017257 Free PMC article. Review.
-
Host-Directed Antiviral Therapy.Clin Microbiol Rev. 2020 May 13;33(3):e00168-19. doi: 10.1128/CMR.00168-19. Print 2020 Jun 17. Clin Microbiol Rev. 2020. PMID: 32404434 Free PMC article. Review.
-
Antifungals: Mechanism of Action and Drug Resistance.Adv Exp Med Biol. 2016;892:327-349. doi: 10.1007/978-3-319-25304-6_14. Adv Exp Med Biol. 2016. PMID: 26721281 Review.
Cited by
-
An overview of the role of Niemann-pick C1 (NPC1) in viral infections and inhibition of viral infections through NPC1 inhibitor.Cell Commun Signal. 2023 Dec 14;21(1):352. doi: 10.1186/s12964-023-01376-x. Cell Commun Signal. 2023. PMID: 38098077 Free PMC article. Review.
-
Exploiting host kinases to combat dengue virus infection and disease.Antiviral Res. 2025 Sep;241:106172. doi: 10.1016/j.antiviral.2025.106172. Epub 2025 May 8. Antiviral Res. 2025. PMID: 40348023 Review.
-
An Antiherpesviral Host-Directed Strategy Based on CDK7 Covalently Binding Drugs: Target-Selective, Picomolar-Dose, Cross-Virus Reactivity.Pharmaceutics. 2024 Jan 23;16(2):158. doi: 10.3390/pharmaceutics16020158. Pharmaceutics. 2024. PMID: 38399219 Free PMC article.
-
Safety and effectiveness of baloxavir marboxil and oseltamivir for influenza in children: a real-world retrospective study in China.Front Pediatr. 2024 Jul 29;12:1418321. doi: 10.3389/fped.2024.1418321. eCollection 2024. Front Pediatr. 2024. PMID: 39135856 Free PMC article.
-
3D Ex vivo tissue platforms to investigate the early phases of influenza a virus- and SARS-CoV-2-induced respiratory diseases.Emerg Microbes Infect. 2022 Dec;11(1):2160-2175. doi: 10.1080/22221751.2022.2117101. Emerg Microbes Infect. 2022. PMID: 36000328 Free PMC article.
References
-
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19) StatPearls Publishing; Treasure Island, FL, USA: 2020. - PubMed
-
- Anand U., Jakhmola S., Indari O., Jha H.C., Chen Z.S., Tripathi V., Pérez de la Lastra J.M. Potential Therapeutic Targets and Vaccine Development for SARS-CoV-2/COVID-19 Pandemic Management: A Review on the Recent Update. Front. Immunol. 2021;12:658519. doi: 10.3389/fimmu.2021.658519. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

