Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study
- PMID: 35215330
- PMCID: PMC8880736
- DOI: 10.3390/ph15020218
Carbocysteine Modifies Circulating miR-21, IL-8, sRAGE, and fAGEs Levels in Mild Acute Exacerbated COPD Patients: A Pilot Study
Abstract
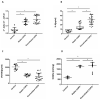
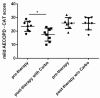
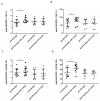
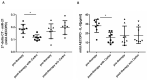
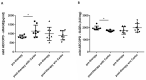
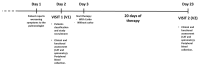
Patients with Chronic Obstructive Pulmonary Disease (COPD) periodically experience acute exacerbation (AECOPD). Carbocysteine represents a valid add on therapy in COPD by exerting antioxidant and anti-inflammatory activities. The in vivo effects of carbocysteine on inflammatory markers are not yet fully understood. The aims of this study were to assess: (i) miR-21, IL-8, soluble Receptor for Advanced Glycation End Products (sRAGE), and fluorescent Advanced Glycation End Products (fAGEs) in control subjects (n = 9), stable (n = 9), and AECOPD patients (n = 24); and (ii) whether carbocysteine modifies these markers and the functional parameters in mild AECOPD patients. Mild AECOPD patients received or not carbocysteine along with background inhalation therapy for 20 days. At the onset and at the end of the observation period, the following parameters were evaluated: FEV1, FEF25-75%, CAT questionnaire; miR-21 by Real Time PCR; IL-8 and sRAGE by ELISA; and fAGEs by spectro-fluorescence method. COPD patients showed higher levels of miR-21, IL-8, fAGEs and lower levels of sRAGE compared to that of controls. miR-21 inversely correlated with FEV1. IL-8 and fAGEs were significantly different in stable and exacerbated COPD patients. Carbocysteine improved symptoms, FEV1 and FEF25-75%, increased sRAGE, and reduced miR-21, IL-8, and fAGEs in mild AECOPD patients. The present study provides compelling evidence that carbocysteine may help to manage mild AECOPD by downregulating some parameters of systemic inflammation.
Keywords: COPD; antioxidants; carbocysteine; exacerbations; inflammation; respiratory pharmacology.
Conflict of interest statement
Elisabetta Pace’s competing interests: she received an unconditional grant by Dompè. This relationship did not influence author’s objectivity. Luigi Lanata is an employee of Dompè. This relationship did not influence authors’ objectivity. All the other authors declare that there is no conflict of interest regarding the publication of this paper.
Figures
References
-
- Singh D., Agusti A., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Criner G.J., Frith P., Halpin D.M.G., Han M., et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD science committee report 2019. Eur. Respir. J. 2019;53:1900164. doi: 10.1183/13993003.00164-2019. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

