The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial
- PMID: 35215453
- PMCID: PMC8880761
- DOI: 10.3390/nu14040805
The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial
Erratum in
-
Correction: Ebrahim et al. The Effect of ß-Glucan Prebiotic on Kidney Function, Uremic Toxins and Gut Microbiome in Stage 3 to 5 Chronic Kidney Disease (CKD) Predialysis Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 805.Nutrients. 2025 Sep 25;17(19):3054. doi: 10.3390/nu17193054. Nutrients. 2025. PMID: 41097262 Free PMC article.
Abstract
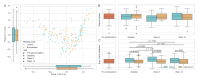
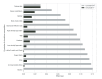
There is growing evidence that gut dysbiosis contributes to the progression of chronic kidney disease (CKD) owing to several mechanisms, including microbiota-derived uremic toxins, diet and immune-mediated factors. The aim of this study was to investigate the effect of a ß-glucan prebiotic on kidney function, uremic toxins and the gut microbiome in stage 3 to 5 CKD participants. Fifty-nine participants were randomized to either the ß-glucan prebiotic intervention group (n = 30) or the control group (n = 29). The primary outcomes were to assess kidney function (urea, creatinine and glomerular filtration rate), plasma levels of total and free levels of uremic toxins (p-cresyl sulfate (pCS), indoxyl-sulfate (IxS), p-cresyl glucuronide (pCG) and indoxyl 3-acetic acid (IAA) and gut microbiota using 16S rRNA sequencing at baseline, week 8 and week 14. The intervention group (age 40.6 ± 11.4 y) and the control group (age 41.3 ± 12.0 y) did not differ in age or any other socio-demographic variables at baseline. There were no significant changes in kidney function over 14 weeks. There was a significant reduction in uremic toxin levels at different time points, in free IxS at 8 weeks (p = 0.003) and 14 weeks (p < 0.001), free pCS (p = 0.006) at 14 weeks and total and free pCG (p < 0.001, p < 0.001, respectively) and at 14 weeks. There were no differences in relative abundances of genera between groups. The redundancy analysis showed a few factors significantly affected the gut microbiome: these included triglyceride levels (p < 0.001), body mass index (p = 0.002), high- density lipoprotein (p < 0.001) and the prebiotic intervention (p = 0.002). The ß-glucan prebiotic significantly altered uremic toxin levels of intestinal origin and favorably affected the gut microbiome.
Keywords: chronic kidney disease (CKD); gut microbiome; prebiotic; uremic toxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bikbov B., Purcell C.A., Levey A.S., Smith M., Abdoli A., Abebe M., Adebayo O.M., Afarideh M., Agarwal S.K., Agudelo-Botero M., et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

