Recent Advances in the Synthesis of Complex Macromolecular Architectures Based on Poly(N-vinyl pyrrolidone) and the RAFT Polymerization Technique
- PMID: 35215614
- PMCID: PMC8880212
- DOI: 10.3390/polym14040701
Recent Advances in the Synthesis of Complex Macromolecular Architectures Based on Poly(N-vinyl pyrrolidone) and the RAFT Polymerization Technique
Abstract
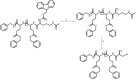
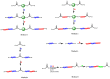
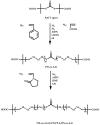
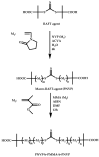
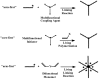
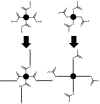
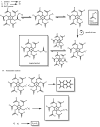
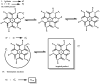
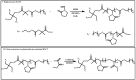
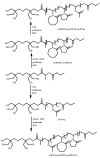
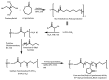
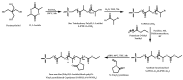
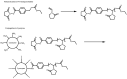
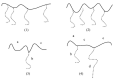
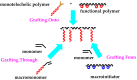
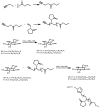
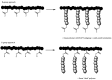
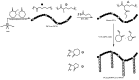
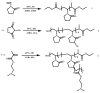
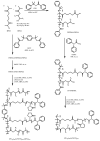
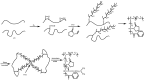
Recent advances in the controlled RAFT polymerization of complex macromolecular architectures based on poly(N-vinyl pyrrolidone), PNVP, are summarized in this review article. Special interest is given to the synthesis of statistical copolymers, block copolymers, and star polymers and copolymers, along with graft copolymers and more complex architectures. In all cases, PNVP is produced via RAFT techniques, whereas other polymerization methods can be employed in combination with RAFT to provide the desired final products. The advantages and limitations of the synthetic methodologies are discussed in detail.
Keywords: RAFT polymerization; block copolymers; graft copolymers; macromolecular architecture; poly(N-vinyl pyrrolidone); star polymers; statistical copolymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





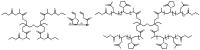
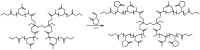

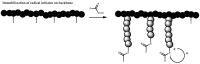
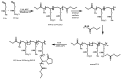
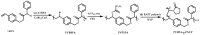
Similar articles
-
Synthesis and Micellization Behavior of Amphiphilic Block Copolymers of Poly(N-vinyl Pyrrolidone) and Poly(Benzyl Methacrylate): Block versus Statistical Copolymers.Polymers (Basel). 2023 May 8;15(9):2225. doi: 10.3390/polym15092225. Polymers (Basel). 2023. PMID: 37177372 Free PMC article.
-
Block Copolymers of Poly(N-Vinyl Pyrrolidone) and Poly(Vinyl Esters) Bearing n-alkyl Side Groups via Reversible Addition-Fragmentation Chain-Transfer Polymerization: Synthesis, Characterization, and Thermal Properties.Polymers (Basel). 2024 Aug 29;16(17):2447. doi: 10.3390/polym16172447. Polymers (Basel). 2024. PMID: 39274080 Free PMC article.
-
Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates.Polymers (Basel). 2025 Apr 21;17(8):1122. doi: 10.3390/polym17081122. Polymers (Basel). 2025. PMID: 40284387 Free PMC article.
-
Using RAFT Polymerization Methodologies to Create Branched and Nanogel-Type Copolymers.Materials (Basel). 2024 Apr 23;17(9):1947. doi: 10.3390/ma17091947. Materials (Basel). 2024. PMID: 38730753 Free PMC article. Review.
-
Amino-acid-based block copolymers by RAFT polymerization.Macromol Rapid Commun. 2012 Jul 13;33(13):1090-107. doi: 10.1002/marc.201100887. Epub 2012 Apr 17. Macromol Rapid Commun. 2012. PMID: 22508409 Review.
Cited by
-
Statistical Copolymers of N-Vinylpyrrolidone and 2-Chloroethyl Vinyl Ether via Radical RAFT Polymerization: Monomer Reactivity Ratios, Thermal Properties, and Kinetics of Thermal Decomposition of the Statistical Copolymers.Polymers (Basel). 2023 Apr 21;15(8):1970. doi: 10.3390/polym15081970. Polymers (Basel). 2023. PMID: 37112117 Free PMC article.
-
Polyester nanoparticles delivering chemotherapeutics: Learning from the past and looking to the future to enhance their clinical impact in tumor therapy.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024 Sep-Oct;16(5):e1990. doi: 10.1002/wnan.1990. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024. PMID: 39217459 Free PMC article. Review.
-
Size-Controlled Ammonium-Based Homopolymers as Broad-Spectrum Antibacterials.Antibiotics (Basel). 2023 Aug 16;12(8):1320. doi: 10.3390/antibiotics12081320. Antibiotics (Basel). 2023. PMID: 37627740 Free PMC article.
-
Synthesis and Micellization Behavior of Amphiphilic Block Copolymers of Poly(N-vinyl Pyrrolidone) and Poly(Benzyl Methacrylate): Block versus Statistical Copolymers.Polymers (Basel). 2023 May 8;15(9):2225. doi: 10.3390/polym15092225. Polymers (Basel). 2023. PMID: 37177372 Free PMC article.
-
Block Copolymers of Poly(N-Vinyl Pyrrolidone) and Poly(Vinyl Esters) Bearing n-alkyl Side Groups via Reversible Addition-Fragmentation Chain-Transfer Polymerization: Synthesis, Characterization, and Thermal Properties.Polymers (Basel). 2024 Aug 29;16(17):2447. doi: 10.3390/polym16172447. Polymers (Basel). 2024. PMID: 39274080 Free PMC article.
References
-
- Teodorescu M., Bercea M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015;549:923–943. doi: 10.1080/03602559.2014.979506. - DOI
-
- Moulay S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013;33:389–443. doi: 10.1515/polyeng-2012-0122. - DOI
-
- Husain M.S.B., Gupta A., Alashwal B.Y., Sharma S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sour. Part A Recovery Util. Environ. Effects. 2018;40:2388–2393. doi: 10.1080/15567036.2018.1495786. - DOI
Publication types
LinkOut - more resources
Full Text Sources

