Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control
- PMID: 35215615
- PMCID: PMC8878160
- DOI: 10.3390/polym14040702
Fabrication of Ropivacaine/Dexamethasone-Eluting Poly(D, L-lactide-co-glycolide) Microparticles via Electrospraying Technique for Postoperational Pain Control
Abstract
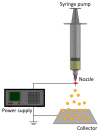
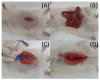
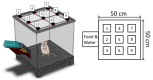
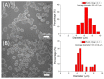
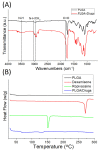
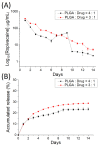
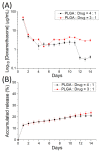
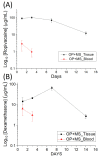
Microencapsulation plays an important role in biomedical technology owing to its particular and attractive characteristics. In this work, we developed ropivacaine and dexamethasone loaded poly(D, L-lactide-co-glycolide) (PLGA) microparticles via electrospraying technique and investigated the release behavior of electrosprayed microparticles. The particle morphology of sprayed particles was assessed using scanning electron microscopy (SEM). The in vitro drug release kinetics were evaluated employing an elution method, and the in vivo pharmaceutical release as well as its efficacy on pain relief were tested using an animal activity model. The microscopic observation suggested that sprayed microparticles exhibit a size distribution of 5-6 µm. Fourier-transform infrared spectrometry and differential scanning calorimetry demonstrated the successful incorporation of pharmaceuticals in the PLGA particulates. The drugs-loaded particles discharged sustainably high concentrations of ropivacaine and dexamethasone at the target region in vivo for over two weeks, and the drug levels in the blood remained low. By adopting the electrospraying technique, we were able to prepare drug-embedded polymeric microparticles with effectiveness and with a sustainable capability for postoperative pain control.
Keywords: dexamethasone; electrospraying; microparticles; ropivacaine; sustained release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lengyel M., Kállai-Szabó N., Antal V., Laki A.J., Antal I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019;87:20. doi: 10.3390/scipharm87030020. - DOI
-
- Jaworek A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007;176:18–35. doi: 10.1016/j.powtec.2007.01.035. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

