New Insights of Scaffolds Based on Hydrogels in Tissue Engineering
- PMID: 35215710
- PMCID: PMC8875010
- DOI: 10.3390/polym14040799
New Insights of Scaffolds Based on Hydrogels in Tissue Engineering
Abstract
In recent years, biomaterials development and characterization for new applications in regenerative medicine or controlled release represent one of the biggest challenges. Tissue engineering is one of the most intensively studied domain where hydrogels are considered optimum applications in the biomedical field. The delicate nature of hydrogels and their low mechanical strength limit their exploitation in tissue engineering. Hence, developing new, stronger, and more stable hydrogels with increased biocompatibility, is essential. However, both natural and synthetic polymers possess many limitations. Hydrogels based on natural polymers offer particularly high biocompatibility and biodegradability, low immunogenicity, excellent cytocompatibility, variable, and controllable solubility. At the same time, they have poor mechanical properties, high production costs, and low reproducibility. Synthetic polymers come to their aid through superior mechanical strength, high reproducibility, reduced costs, and the ability to regulate their composition to improve processes such as hydrolysis or biodegradation over variable periods. The development of hydrogels based on mixtures of synthetic and natural polymers can lead to the optimization of their properties to obtain ideal scaffolds. Also, incorporating different nanoparticles can improve the hydrogel's stability and obtain several biological effects. In this regard, essential oils and drug molecules facilitate the desired biological effect or even produce a synergistic effect. This study's main purpose is to establish the main properties needed to develop sustainable polymeric scaffolds. These scaffolds can be applied in tissue engineering to improve the tissue regeneration process without producing other side effects to the environment.
Keywords: healing process; hydrogels; polymers; regeneration; scaffolds; tissue engineering.
Conflict of interest statement
The author Denisa-Maria Radulescu is an employee of MDPI, however, she does not work for the journal Polymers at the time of submission and publication. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures








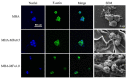
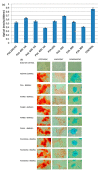
References
-
- Khalil A.M. Interpenetrating polymeric hydrogels as favorable materials for hygienic applications. Biointerface Res. Appl. Chem. 2020;10:5011–5020. doi: 10.33263/briac102.011020. - DOI
-
- Tran H.D.N., Park K.D., Ching Y.C., Huynh C., Nguyen D.H. A comprehensive review on polymeric hydrogel and its composite: Matrices of choice for bone and cartilage tissue engineering. J. Ind. Eng. Chem. 2020;89:58–82. doi: 10.1016/j.jiec.2020.06.017. - DOI
-
- Nezhad-Mokhtari P., Ghorbani M., Roshangar L., Soleimani Rad J. A review on the construction of hydrogel scaffolds by various chemically techniques for tissue engineering. Eur. Polym. J. 2019;117:64–76. doi: 10.1016/j.eurpolymj.2019.05.004. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

