Flame Retardancy and Thermal Degradation Behaviors of Thiol-Ene Composites Containing a Novel Phosphorus and Silicon-Containing Flame Retardant
- PMID: 35215733
- PMCID: PMC8962985
- DOI: 10.3390/polym14040820
Flame Retardancy and Thermal Degradation Behaviors of Thiol-Ene Composites Containing a Novel Phosphorus and Silicon-Containing Flame Retardant
Abstract
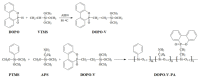
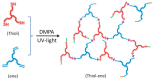
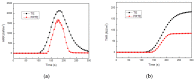
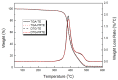
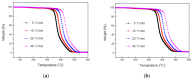
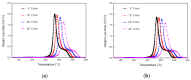
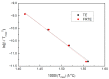
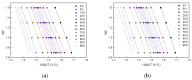
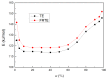
In this article, a novel phosphorus and silicon-containing flame retardant (DOPO-V-PA) was synthesized via condensation reaction and then added into thiol-ene (TE) to prepare a flame-retardant composite. The results of cone calorimeter measurement demonstrated that, compared with pure TE, 22.7% and 53.2% reduction of TE/DOPO-V-PA (thiol-ene/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-vinyltrimethoxysilane-phenyltrimethoxysilane-(3-aminopropyl)trimethoxysilane copolymer) was found for the peak heat release rate (PHRR) and total heat release (THR), respectively. The thermal degradation of TE composites was investigated by the TGA measurement under non-isothermal conditions, and kinetic parameters were both calculated by the Kissinger and Flynn-Wall-Ozawa methods. It was indicated that the activation energies of TE at conversions exceeding 50% were enhanced by the incorporation of DOPO-V-PA for the whole conversion range.
Keywords: activation energy; flame retardancy; kinetics; thermal degradation; thiol-ene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wang X., Wang X., Song L., Xing W., Tang G., Hua W., Hu Y. Preparation and thermal stability of UV-cured epoxy-based coatings modified with octamercaptopropyl POSS. Thermochim. Acta. 2013;568:130–139. doi: 10.1016/j.tca.2013.06.038. - DOI
-
- Yao H.-Y., Lin H.-R., Sue G.-P., Lin Y.-J. Chitosan-based hydrogels prepared by UV polymerization for wound dressing. Polym. Polym. Compos. 2019;27:155–167. doi: 10.1177/0967391118820477. - DOI
-
- Qian J., Li Z., Huang N., Lu Q., Xia J. UV-Irradiation Polymerization of Bis-EDOT Methane Derivatives and Their Appli-cation for Br2 Detection. Polymer. 2021;226:123808. doi: 10.1016/j.polymer.2021.123808. - DOI
-
- Bayramoglu G., Kahraman M., Kayaman-Apohan N., Gungor A. Synthesis and characterization of UV-curable dual hybrid oligomers based on epoxy acrylate containing pendant alkoxysilane groups. Prog. Org. Coat. 2006;57:50–55. doi: 10.1016/j.porgcoat.2006.06.002. - DOI
-
- Kwisnek L., Heinz S., Wiggins J.S., Nazarenko S. Multifunctional thiols as additives in UV-cured PEG-diacrylate membranes for CO2 separation. J. Membr. Sci. 2011;369:429–436. doi: 10.1016/j.memsci.2010.12.022. - DOI
Grants and funding
- 2019A610032/Ningbo Natural Science Foundation
- 201811058002/National Undergraduate Training Program for Innovation and Entrepreneurship
- 2021009/Chongben Foundation
- GZKF202127/the Foundation of State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology, Shandong Academy of Sciences
LinkOut - more resources
Full Text Sources

