Distinct Outcomes in COVID-19 Patients with Positive or Negative RT-PCR Test
- PMID: 35215772
- PMCID: PMC8874612
- DOI: 10.3390/v14020175
Distinct Outcomes in COVID-19 Patients with Positive or Negative RT-PCR Test
Abstract
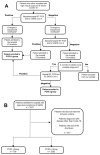
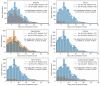
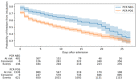
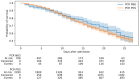
Identification of the SARS-CoV-2 virus by RT-PCR from a nasopharyngeal swab sample is a common test for diagnosing COVID-19. However, some patients present clinical, laboratorial, and radiological evidence of COVID-19 infection with negative RT-PCR result(s). Thus, we assessed whether positive results were associated with intubation and mortality. This study was conducted in a Brazilian tertiary hospital from March to August of 2020. All patients had clinical, laboratory, and radiological diagnosis of COVID-19. They were divided into two groups: positive (+) RT-PCR group, with 2292 participants, and negative (-) RT-PCR group, with 706 participants. Patients with negative RT-PCR testing and an alternative most probable diagnosis were excluded from the study. The RT-PCR(+) group presented increased risk of intensive care unit (ICU) admission, mechanical ventilation, length of hospital stay, and 28-day mortality, when compared to the RT-PCR(-) group. A positive SARS-CoV-2 RT-PCR result was independently associated with intubation and 28 day in-hospital mortality. Accordingly, we concluded that patients with a COVID-19 diagnosis based on clinical data, despite a negative RT-PCR test from nasopharyngeal samples, presented more favorable outcomes than patients with positive RT-PCR test(s).
Keywords: COVID-19; COVID-19 testing; SARS-CoV-2; hospital mortality; intubation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Brandão Neto R.A., Marchini J.F., Marino L.O., Alencar J.C., Lazar Neto F., Ribeiro S., Salvetti F.V., Rahhal H., Gomez Gomez L.M., Bueno C.G., et al. Mortality and other outcomes of patients with coronavirus disease pneumonia admitted to the emergency department: A prospective observational Brazilian study. PLoS ONE. 2021;16:e0244532. - PMC - PubMed
-
- Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. - DOI - PMC - PubMed
-
- World Health Organization (WHO) Clinical Management of COVID-19—Interim Guidance. World Health Organization; Geneva, Switzerland: May 27, 2020. [(accessed on 6 January 2022)]. Available online: https://apps.who.int/iris/handle/10665/332196.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

