The Reservoir of Persistent Human Papillomavirus Infection; Strategies for Elimination Using Anti-Viral Therapies
- PMID: 35215808
- PMCID: PMC8876702
- DOI: 10.3390/v14020214
The Reservoir of Persistent Human Papillomavirus Infection; Strategies for Elimination Using Anti-Viral Therapies
Abstract
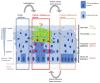
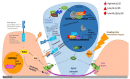
Human Papillomaviruses have co-evolved with their human host, with each of the over 200 known HPV types infecting distinct epithelial niches to cause diverse disease pathologies. Despite the success of prophylactic vaccines in preventing high-risk HPV infection, the development of HPV anti-viral therapies has been hampered by the lack of enzymatic viral functions, and by difficulties in translating the results of in vitro experiments into clinically useful treatment regimes. In this review, we discuss recent advances in anti-HPV drug development, and highlight the importance of understanding persistent HPV infections for future anti-viral design. In the infected epithelial basal layer, HPV genomes are maintained at a very low copy number, with only limited viral gene expression; factors which allow them to hide from the host immune system. However, HPV gene expression confers an elevated proliferative potential, a delayed commitment to differentiation, and preferential persistence of the infected cell in the epithelial basal layer, when compared to their uninfected neighbours. To a large extent, this is driven by the viral E6 protein, which functions in the HPV life cycle as a modulator of epithelial homeostasis. By targeting HPV gene products involved in the maintenance of the viral reservoir, there appears to be new opportunities for the control or elimination of chronic HPV infections.
Keywords: HPV; anti-viral therapy; basal epithelial homeostasis; persistent infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bordignon V., Di Domenico E.G., Trento E., D’agosto G., Cavallo I., Pontone M., Pimpinelli F., Mariani L., Ensoli F. How Human Papillomavirus Replication and Immune Evasion Strategies Take Advantage of the Host DNA Damage Repair Machinery. Viruses. 2017;9:390. doi: 10.3390/v9120390. - DOI - PMC - PubMed
-
- Bruni L., Diaz M., Barrionuevo-Rosas L., Herrero R., Bray F., Bosch F.X., de Sanjosé S., Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health. 2016;4:e453–e463. doi: 10.1016/S2214-109X(16)30099-7. - DOI - PubMed
-
- De Vuyst H., Mugo N.R., Franceschi S., McKenzie K., Tenet V., Njoroge J., Rana F.S., Sakr S.R., Snijders P.J.F., Chung M. Residual Disease and HPV Persistence after Cryotherapy for Cervical Intraepithelial Neoplasia Grade 2/3 in HIV-Positive Women in Kenya. PLoS ONE. 2014;9:e111037. doi: 10.1371/journal.pone.0111037. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

