Time-Dependent Proinflammatory Responses Shape Virus Interference during Coinfections of Influenza A Virus and Influenza D Virus
- PMID: 35215819
- PMCID: PMC8878573
- DOI: 10.3390/v14020224
Time-Dependent Proinflammatory Responses Shape Virus Interference during Coinfections of Influenza A Virus and Influenza D Virus
Abstract
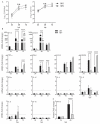
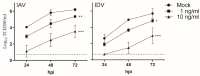
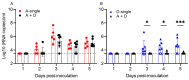
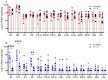
Both influenza A virus (IAV) and influenza D virus (IDV) are enzootic in pigs. IAV causes approximately 100% morbidity with low mortality, whereas IDV leads to only mild respiratory diseases in pigs. In this study, we performed a series of coinfection experiments in vitro and in vivo to understand how IAV and IDV interact and cause pathogenesis during coinfection. The results showed that IAV inhibited IDV replication when infecting swine tracheal epithelial cells (STECs) with IAV 24 or 48 h prior to IDV inoculation and that IDV suppressed IAV replication when IDV preceded IAV inoculation by 48 h. Virus interference was not identified during simultaneous IAV/IDV infections or with 6 h between the two viral infections, regardless of their order. The interference pattern at 24 and 48 h correlated with proinflammatory responses induced by the first infection, which, for IDV, was slower than for IAV by about 24 h. The viruses did not interfere with each other if both infected the cells before proinflammatory responses were induced. Coinfection in pigs further demonstrated that IAV interfered with both viral shedding and virus replication of IDV, especially in the upper respiratory tract. Clinically, coinfection of IDV and IAV did not show significant enhancement of disease pathogenesis, compared with the pigs infected with IAV alone. In summary, this study suggests that interference during coinfection of IAV and IDV is primarily due to the proinflammatory response; therefore, it is dependent on the time between infections and the order of infection. This study facilitates our understanding of virus epidemiology and pathogenesis associated with IAV and IDV coinfection.
Keywords: co-infection; influenza A virus; influenza D virus; proinflammatory response; swine; viral interference.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Palese P., Shaw M. Orthomyxoviridae: The Viruses and Their Replication. In: Knipe D., Howley P., editors. Fields Virology. 5th ed. Volume 2. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1647–1690.
-
- Hause B.M., Ducatez M., Collin E.A., Ran Z., Liu R., Sheng Z., Armien A., Kaplan B., Chakravarty S., Hoppe A.D., et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013;9:e1003176. doi: 10.1371/journal.ppat.1003176. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

