Characterization of a Broadly Neutralizing Monoclonal Antibody against SARS-CoV-2 Variants
- PMID: 35215823
- PMCID: PMC8878391
- DOI: 10.3390/v14020230
Characterization of a Broadly Neutralizing Monoclonal Antibody against SARS-CoV-2 Variants
Abstract
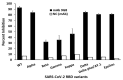
The constant mutation of SARS-CoV-2 has led to the emergence of new variants, which call for urgent effective therapeutic interventions. The trimeric spike (S) protein of SARS-CoV-2 is highly immunogenic with the receptor-binding domain (RBD) that binds first to the cellular receptor angiotensin-converting enzyme 2 (ACE2) and is therefore the target of many neutralizing antibodies. In this study, we characterized a broadly neutralizing monoclonal antibody (mAb) 9G8, which shows potent neutralization against the authentic SARS-CoV-2 wild-type (WT), Alpha (B.1.1.7), and Delta (1.617.2) viruses. Furthermore, mAb 9G8 also displayed a prominent neutralizing efficacy in the SARS-CoV-2 surrogate virus neutralization test (sVNT) against the Epsilon (B.1.429/7), Kappa (B.1.617.1), Gamma (P.1), Beta (B.1.351), and Delta Plus (1.617.2.1) RBD variants in addition to the variants mentioned above. Based on our in vitro escape mutant studies, we proved that the mutations V483F and Y489H within the RBD were involved in ACE2 binding and caused the neutralizing evasion of the virus from mAb 9G8. The development of such a cross-reactive neutralizing antibody against majority of the SARS-CoV-2 variants provides an important insight into pursuing future therapeutic agents for the prevention and treatment of COVID-19.
Keywords: SARS-CoV-2 variants; cross-neutralizing antibody; escape mutant; neutralizing epitope; spike RBD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Characterization of MW06, a human monoclonal antibody with cross-neutralization activity against both SARS-CoV-2 and SARS-CoV.MAbs. 2021 Jan-Dec;13(1):1953683. doi: 10.1080/19420862.2021.1953683. MAbs. 2021. PMID: 34313527 Free PMC article.
-
Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants.Elife. 2020 Oct 28;9:e61312. doi: 10.7554/eLife.61312. Elife. 2020. PMID: 33112236 Free PMC article.
-
Neutralization potency of monoclonal antibodies recognizing dominant and subdominant epitopes on SARS-CoV-2 Spike is impacted by the B.1.1.7 variant.Immunity. 2021 Jun 8;54(6):1276-1289.e6. doi: 10.1016/j.immuni.2021.03.023. Epub 2021 Apr 1. Immunity. 2021. PMID: 33836142 Free PMC article.
-
Mutations in the SARS-CoV-2 spike receptor binding domain and their delicate balance between ACE2 affinity and antibody evasion.Protein Cell. 2024 May 28;15(6):403-418. doi: 10.1093/procel/pwae007. Protein Cell. 2024. PMID: 38442025 Free PMC article. Review.
-
Analysis of the molecular mechanism of SARS-CoV-2 antibodies.Biochem Biophys Res Commun. 2021 Aug 20;566:45-52. doi: 10.1016/j.bbrc.2021.06.001. Epub 2021 Jun 5. Biochem Biophys Res Commun. 2021. PMID: 34116356 Free PMC article. Review.
Cited by
-
Long-Term Vaccination and Treatment Strategies for COVID-19 Disease and Future Coronavirus Pandemics.Adv Exp Med Biol. 2023;1412:27-49. doi: 10.1007/978-3-031-28012-2_2. Adv Exp Med Biol. 2023. PMID: 37378760 Review.
References
-
- Wibmer C.K., Ayres F., Hermanus T., Madzivhandila M., Kgagudi P., Oosthuysen B., Lambson B.E., de Oliveira T., Vermeulen M., van der Berg K., et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021;27:622–625. doi: 10.1038/s41591-021-01285-x. - DOI - PubMed
-
- Chen R.E., Zhang X., Case J.B., Winkler E.S., Liu Y., VanBlargan L.A., Liu J., Errico J.M., Xie X., Suryadevara N., et al. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat. Med. 2021;27:717–726. doi: 10.1038/s41591-021-01294-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

