Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS)
- PMID: 35215843
- PMCID: PMC8879837
- DOI: 10.3390/v14020250
Involvement of Th1Th17 Cell Subpopulations in the Immune Responses of Mothers Who Gave Birth to Children with Congenital Zika Syndrome (CZS)
Abstract
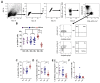
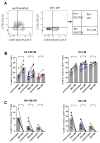
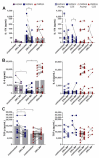
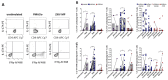
High levels of T helper 17 cell (Th17)-related cytokines have been shown in acute Zika virus (ZIKV) infection. We hypothesized that the high levels of Th17-related cytokines, associated with a regulatory environment during pregnancy, create a favorable milieu for the differentiation of CD4+Th17 cells. We present data from a cross-sectional study on mothers who confirmed ZIKV infection by qRT-PCR and their children. We also recruited non-pregnant women infected with ZIKV in the same period. ZIKV infection occurred between 2015 and 2017. We collected samples for this study between 2018 and 2019, years after the initial infection. We highlight that, after in vitro stimulation with ZIKV CD4 megapool (ZIKV MP), we found a lower frequency of IL-17-producing CD4+ T cells (Th17), especially in the mothers, confirmed by the decrease in IL-17 production in the supernatant. However, a higher frequency of CD4+ IL-17+ IFN-γ+ T cells (Th1Th17) responding to the ZIKV MP was observed in the cells of the mothers and children but not in those of the non-pregnant women. Our data indicate that the priming of CD4 T cells of the Th1Th17 phenotype occurred preferentially in the mothers who gave birth to children with CZS and in the children.
Keywords: T cells; Th17 cells; Zika; congenital Zika syndrome (CZS); pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pan American Health Organization. World Health Organization Epidemiological Update: Neurological Syndrome, Congenital Anomalies and Zika Virus Infection. Washinton, DC. 2016. [(accessed on 3 June 2020)]. Available online: https://www.paho.org/hq/dmdocuments/2016/2016-jan-17-cha-epi-update-zika....
-
- Ministry of Health of Brazil. Health Surveillance Department Protocolo de Vigilância e Resposta À Microcefalia Relacionada À Infecção Pelo Vírus Zika. [(accessed on 3 June 2020)];2015 Available online: https://pesquisa.bvsalud.org/gim/resource/en/lil-773183.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

