Rapid Biosensor of SARS-CoV-2 Using Specific Monoclonal Antibodies Recognizing Conserved Nucleocapsid Protein Epitopes
- PMID: 35215848
- PMCID: PMC8879994
- DOI: 10.3390/v14020255
Rapid Biosensor of SARS-CoV-2 Using Specific Monoclonal Antibodies Recognizing Conserved Nucleocapsid Protein Epitopes
Abstract
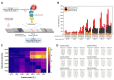
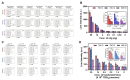
Coronavirus disease 2019 (COVID-19), the pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by symptoms such as fever, fatigue, a sore throat, diarrhea, and coughing. Although various new vaccines against COVID-19 have been developed, early diagnostics, isolation, and prevention remain important due to virus mutations resulting in rapid and high disease transmission. Amino acid substitutions in the major diagnostic target antigens of SARS-CoV-2 may lower the sensitivity for the detection of SARS-CoV-2. For this reason, we developed specific monoclonal antibodies that bind to epitope peptides as antigens for the rapid detection of SARS-CoV-2 NP. The binding affinity between antigenic peptides and monoclonal antibodies was investigated, and a sandwich pair for capture and detection was employed to develop a rapid biosensor for SARS-CoV-2 NP. The rapid biosensor, based on a monoclonal antibody pair binding to conserved epitopes of SARS-CoV-2 NP, detected cultured virus samples of SARS-CoV-2 (1.4 × 103 TCID50/reaction) and recombinant NP (1 ng/mL). Laboratory confirmation of the rapid biosensor was performed with clinical specimens (n = 16) from COVID-19 patients and other pathogens. The rapid biosensor consisting of a monoclonal antibody pair (75E12 for capture and the 54G6/54G10 combination for detection) binding to conserved epitopes of SARS-CoV-2 NP could assist in the detection of SARS-CoV-2 NP under the circumstance of spreading SARS-CoV-2 variants.
Keywords: COVID-19; SARS-CoV-2; biosensor; conserved epitope; monoclonal antibody; nucleocapsid; virus mutation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Monoclonal Antibodies against Nucleocapsid Protein of SARS-CoV-2 Variants for Detection of COVID-19.Int J Mol Sci. 2021 Nov 17;22(22):12412. doi: 10.3390/ijms222212412. Int J Mol Sci. 2021. PMID: 34830291 Free PMC article.
-
Development of a double-antibody sandwich ELISA for detection of SARS-CoV-2 variants based on nucleocapsid protein-specific antibodies.Microbiol Immunol. 2024 Nov;68(11):393-398. doi: 10.1111/1348-0421.13173. Epub 2024 Sep 17. Microbiol Immunol. 2024. PMID: 39287179
-
Characterization and application of a series of monoclonal antibodies against SARS-CoV-2 nucleocapsid protein.J Med Virol. 2023 Jan;95(1):e28225. doi: 10.1002/jmv.28225. Epub 2022 Oct 25. J Med Virol. 2023. PMID: 36238992 Free PMC article.
-
Characterization of conformation-specific monoclonal antibodies against rabies virus nucleoprotein.Arch Virol. 2010 Aug;155(8):1187-92. doi: 10.1007/s00705-010-0709-x. Epub 2010 Jun 4. Arch Virol. 2010. PMID: 20521069 Free PMC article. Review.
-
New approach in SARS-CoV-2 surveillance using biosensor technology: a review.Environ Sci Pollut Res Int. 2022 Jan;29(2):1677-1695. doi: 10.1007/s11356-021-17096-z. Epub 2021 Oct 23. Environ Sci Pollut Res Int. 2022. PMID: 34689274 Free PMC article. Review.
Cited by
-
A Protein Microarray-Based Respiratory Viral Antigen Testing Platform for COVID-19 Surveillance.Biomedicines. 2022 Sep 9;10(9):2238. doi: 10.3390/biomedicines10092238. Biomedicines. 2022. PMID: 36140339 Free PMC article.
-
Assessment of Immunogenic and Antigenic Properties of Recombinant Nucleocapsid Proteins of Five SARS-CoV-2 Variants in a Mouse Model.Viruses. 2023 Jan 13;15(1):230. doi: 10.3390/v15010230. Viruses. 2023. PMID: 36680269 Free PMC article.
-
Rapid assays of SARS-CoV-2 virus and noble biosensors by nanomaterials.Nano Converg. 2024 Jan 8;11(1):2. doi: 10.1186/s40580-023-00408-z. Nano Converg. 2024. PMID: 38190075 Free PMC article. Review.
-
State of the art in epitope mapping and opportunities in COVID-19.Future Sci OA. 2023 Feb;16(3-06):FSO832. doi: 10.2144/fsoa-2022-0048. Epub 2023 Mar 6. Future Sci OA. 2023. PMID: 36897962 Free PMC article. Review.
-
Progress in Biosensors for the Point-of-Care Diagnosis of COVID-19.Sensors (Basel). 2022 Sep 29;22(19):7423. doi: 10.3390/s22197423. Sensors (Basel). 2022. PMID: 36236521 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

