Fifty Shades of Erns: Innate Immune Evasion by the Viral Endonucleases of All Pestivirus Species
- PMID: 35215858
- PMCID: PMC8880635
- DOI: 10.3390/v14020265
Fifty Shades of Erns: Innate Immune Evasion by the Viral Endonucleases of All Pestivirus Species
Abstract
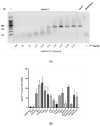
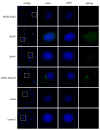
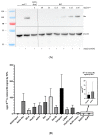
The genus Pestivirus, family Flaviviridae, includes four historically accepted species, i.e., bovine viral diarrhea virus (BVDV)-1 and -2, classical swine fever virus (CSFV), and border disease virus (BDV). A large number of new pestivirus species were identified in recent years. A common feature of most members is the presence of two unique proteins, Npro and Erns, that pestiviruses evolved to regulate the host's innate immune response. In addition to its function as a structural envelope glycoprotein, Erns is also released in the extracellular space, where it is endocytosed by neighboring cells. As an endoribonuclease, Erns is able to cleave viral ss- and dsRNAs, thus preventing the stimulation of the host's interferon (IFN) response. Here, we characterize the basic features of soluble Erns of a large variety of classified and unassigned pestiviruses that have not yet been described. Its ability to form homodimers, its RNase activity, and the ability to inhibit dsRNA-induced IFN synthesis were investigated. Overall, we found large differences between the various Erns proteins that cannot be predicted solely based on their primary amino acid sequences, and that might be the consequence of different virus-host co-evolution histories. This provides valuable information to delineate the structure-function relationship of pestiviral endoribonucleases.
Keywords: atypical pestiviruses; bovine viral diarrhea virus (BVDV); innate immune evasion; interferon type-I; pestivirus; viral RNase; viral endonuclease.
Conflict of interest statement
The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Positively Charged Amino Acids in the Pestiviral Erns Control Cell Entry, Endoribonuclease Activity and Innate Immune Evasion.Viruses. 2021 Aug 10;13(8):1581. doi: 10.3390/v13081581. Viruses. 2021. PMID: 34452446 Free PMC article.
-
Prolonged activity of the pestiviral RNase Erns as an interferon antagonist after uptake by clathrin-mediated endocytosis.J Virol. 2014 Jul;88(13):7235-43. doi: 10.1128/JVI.00672-14. Epub 2014 Apr 16. J Virol. 2014. PMID: 24741078 Free PMC article.
-
The viral RNase E(rns) prevents IFN type-I triggering by pestiviral single- and double-stranded RNAs.Virus Res. 2009 Mar;140(1-2):15-23. doi: 10.1016/j.virusres.2008.10.015. Epub 2008 Dec 16. Virus Res. 2009. PMID: 19041350
-
What can pestiviral endonucleases teach us about innate immunotolerance?Cytokine Growth Factor Rev. 2016 Jun;29:53-62. doi: 10.1016/j.cytogfr.2016.03.003. Epub 2016 Mar 17. Cytokine Growth Factor Rev. 2016. PMID: 27021825 Free PMC article. Review.
-
Current progress on innate immune evasion mediated by Npro protein of pestiviruses.Front Immunol. 2023 Apr 5;14:1136051. doi: 10.3389/fimmu.2023.1136051. eCollection 2023. Front Immunol. 2023. PMID: 37090696 Free PMC article. Review.
Cited by
-
Immune evasion strategies of bovine viral diarrhea virus.Front Cell Infect Microbiol. 2023 Oct 13;13:1282526. doi: 10.3389/fcimb.2023.1282526. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37900320 Free PMC article. Review.
-
Pestiviruses infection: Interferon-virus mutual regulation.Front Cell Infect Microbiol. 2023 Mar 2;13:1146394. doi: 10.3389/fcimb.2023.1146394. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36936761 Free PMC article. Review.
-
Exclusion of Superinfection or Enhancement of Superinfection in Pestiviruses-APPV Infection Is Not Dependent on ADAM17.Viruses. 2024 Nov 26;16(12):1834. doi: 10.3390/v16121834. Viruses. 2024. PMID: 39772144 Free PMC article.
-
Assessment of the Safety Profile of Chimeric Marker Vaccine against Classical Swine Fever: Reversion to Virulence Study.Viruses. 2024 Jul 12;16(7):1120. doi: 10.3390/v16071120. Viruses. 2024. PMID: 39066282 Free PMC article.
-
Characterization of the First Marine Pestivirus, Phocoena Pestivirus (PhoPeV).Viruses. 2025 Jan 14;17(1):107. doi: 10.3390/v17010107. Viruses. 2025. PMID: 39861896 Free PMC article.
References
-
- King A.M.Q., Lefkowitz E.J., Mushegian A.R., Adams M.J., Dutilh B.E., Gorbalenya A.E., Harrach B., Harrison R.L., Junglen S., Knowles N.J., et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018) Arch. Virol. 2018;163:2601–2631. doi: 10.1007/s00705-018-3847-1. - DOI - PubMed
-
- Meyers G., Ege A., Fetzer C., von Freyburg M., Elbers K., Carr V., Prentice H., Charleston B., Schürmann E.-M. Bovine viral diarrhea virus: Prevention of persistent fetal infection by a combination of two mutations affecting Erns RNase and Npro protease. J. Virol. 2007;81:3327–3338. doi: 10.1128/JVI.02372-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

