The Spread of SARS-CoV-2 Variant Omicron with a Doubling Time of 2.0-3.3 Days Can Be Explained by Immune Evasion
- PMID: 35215887
- PMCID: PMC8875689
- DOI: 10.3390/v14020294
The Spread of SARS-CoV-2 Variant Omicron with a Doubling Time of 2.0-3.3 Days Can Be Explained by Immune Evasion
Abstract
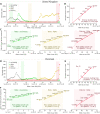
Omicron, the novel highly mutated SARS-CoV-2 Variant of Concern (VOC, Pango lineage B.1.1.529) was first collected in early November 2021 in South Africa. By the end of November 2021, it had spread and approached fixation in South Africa, and had been detected on all continents. We analyzed the exponential growth of Omicron over four-week periods in the two most populated of South Africa's provinces, Gauteng and KwaZulu-Natal, arriving at the doubling time estimates of, respectively, 3.3 days (95% CI: 3.2-3.4 days) and 2.7 days (95% CI: 2.3-3.3 days). Similar or even shorter doubling times were observed in other locations: Australia (3.0 days), New York State (2.5 days), UK (2.4 days), and Denmark (2.0 days). Log-linear regression suggests that the spread began in Gauteng around 11 October 2021; however, due to presumable stochasticity in the initial spread, this estimate can be inaccurate. Phylogenetics-based analysis indicates that the Omicron strain started to diverge between 6 October and 29 October 2021. We estimated that the weekly growth of the ratio of Omicron to Delta is in the range of 7.2-10.2, considerably higher than the growth of the ratio of Delta to Alpha (estimated to be in in the range of 2.5-4.2), and Alpha to pre-existing strains (estimated to be in the range of 1.8-2.7). High relative growth does not necessarily imply higher Omicron infectivity. A two-strain SEIR model suggests that the growth advantage of Omicron may stem from immune evasion, which permits this VOC to infect both recovered and fully vaccinated individuals. As we demonstrated within the model, immune evasion is more concerning than increased transmissibility, because it can facilitate larger epidemic outbreaks.
Keywords: COVID-19 pandemic; Omicron variant; SARS-CoV-2; genome sequencing; mutation.
Conflict of interest statement
The authors have no competing interest.
Figures



References
-
- Implications of the Further Emergence and Spread of the SARS-CoV-2 B.1.1.529 Variant of Concern (Omicron) for the EU/EEA—First Update. [(accessed on 2 December 2021)]. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-threat-assessme....
-
- Madhi S.A. South African Population Immunity and Severe Covid-19 with Omicron Variant. medRxiv. 2021 doi: 10.1101/2021.12.20.21268096. - DOI
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

