Immunogenicity Following Administration of BNT162b2 and Ad26.COV2.S COVID-19 Vaccines in the Pregnant Population during the Third Trimester
- PMID: 35215900
- PMCID: PMC8878278
- DOI: 10.3390/v14020307
Immunogenicity Following Administration of BNT162b2 and Ad26.COV2.S COVID-19 Vaccines in the Pregnant Population during the Third Trimester
Abstract
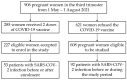
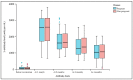
Globally, COVID-19 vaccines are currently being used to prevent transmission and to reduce morbidity and death associated with SARS-CoV-2 infection. Current research reveals that vaccines such as BNT162b2 and Ad26.COV2.S are highly immunogenic and have high short-term effectiveness for most of the known viral variants. Clinical trials showed satisfying results in the general population, but the reluctance in testing and vaccinating pregnant women left this category with little evidence regarding the safety, efficacy, and immunogenicity following COVID-19 vaccination. With the worldwide incidence of COVID-19 remaining high and the possibility of new transmissible SARS-CoV-2 mutations, data on vaccination effectiveness and antibody dynamics in pregnant patients are critical for determining the need for special care or further booster doses. An observational study was developed to evaluate pregnant women receiving the complete COVID-19 vaccination scheme using the BNT162b2 and Ad26.COV2.S, and determine pregnancy-related outcomes in the mothers and their newborns, as well as determining adverse events after vaccination and immunogenicity of vaccines during four months. There were no abnormal findings in pregnancy and newborn characteristics comparing vaccinated versus unvaccinated pregnant women. COVID-19 seropositive pregnant women had significantly higher spike antibody titers than seronegative patients with similar characteristics, although they were more likely to develop fever and lymphadenopathy following vaccination. The same group of pregnant women showed no statistically significant differences in antibody titers during a 4-month period when compared with case-matched non-pregnant women. The BNT162b2 and Ad26.COV2.S vaccines are safe to administer during the third trimester of pregnancy, while their safety, efficacy, and immunogenicity remain similar to those of the general population.
Keywords: Ad26.COV2.S; BNT162b2; COVID-19; SARS-CoV-2; pregnancy vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming.N Engl J Med. 2022 Mar 10;386(10):951-963. doi: 10.1056/NEJMoa2116747. Epub 2022 Jan 19. N Engl J Med. 2022. PMID: 35045226 Free PMC article. Clinical Trial.
-
Pre- and post-Ad26.COV2·S booster dose antibody levels predict COVID-19 disease risk.Vaccine. 2024 Sep 17;42(22):126159. doi: 10.1016/j.vaccine.2024.126159. Epub 2024 Aug 8. Vaccine. 2024. PMID: 39121698 Clinical Trial.
-
Homologous and Heterologous Covid-19 Booster Vaccinations.N Engl J Med. 2022 Mar 17;386(11):1046-1057. doi: 10.1056/NEJMoa2116414. Epub 2022 Jan 26. N Engl J Med. 2022. PMID: 35081293 Free PMC article. Clinical Trial.
-
Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: a scoping review.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD015021. doi: 10.1002/14651858.CD015021. Cochrane Database Syst Rev. 2022. PMID: 35943061 Free PMC article.
-
Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine.Immunol Rev. 2022 Sep;310(1):47-60. doi: 10.1111/imr.13088. Epub 2022 Jun 11. Immunol Rev. 2022. PMID: 35689434 Free PMC article. Review.
Cited by
-
Effect of the COVID-19 vaccination on feto-maternal outcomes: A prospective cohort study among Indian pregnant women.Indian J Med Res. 2024 Sep&Oct;160(3&4):371-378. doi: 10.25259/IJMR_1014_2024. Indian J Med Res. 2024. PMID: 39632631 Free PMC article.
-
Assessing the Impact of COVID-19 Vaccination on Preterm Birth: A Systematic Review with Meta-Analysis.Vaccines (Basel). 2024 Jan 19;12(1):102. doi: 10.3390/vaccines12010102. Vaccines (Basel). 2024. PMID: 38276674 Free PMC article. Review.
-
Laboratory Findings and Clinical Outcomes of ICU-admitted COVID-19 Patients: A Retrospective Assessment of Particularities Identified among Romanian Minorities.J Pers Med. 2023 Jan 21;13(2):195. doi: 10.3390/jpm13020195. J Pers Med. 2023. PMID: 36836429 Free PMC article.
-
Characterization and Outcomes of SARS-CoV-2 Infection in Overweight and Obese Patients: A Dynamic Comparison of COVID-19 Pandemic Waves.J Clin Med. 2022 May 21;11(10):2916. doi: 10.3390/jcm11102916. J Clin Med. 2022. PMID: 35629042 Free PMC article.
-
Appraisal of COVID-19 Vaccination Acceptance in the Romanian Pregnant Population.Vaccines (Basel). 2022 Jun 15;10(6):952. doi: 10.3390/vaccines10060952. Vaccines (Basel). 2022. PMID: 35746560 Free PMC article.
References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 10 December 2021)]. Available online: https://covid19.who.int.
-
- United States Food and Drug Administration Moderna COVID-19 Vaccine. [(accessed on 10 December 2021)];2020 Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
-
- United States Food and Drug Administration Comirnaty and Pfizer-BioNTech COVID-19 Vaccine 2020. [(accessed on 10 December 2021)]; Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

