Salmon Erythrocytes Sequester Active Virus Particles in Infectious Salmon Anaemia
- PMID: 35215905
- PMCID: PMC8879071
- DOI: 10.3390/v14020310
Salmon Erythrocytes Sequester Active Virus Particles in Infectious Salmon Anaemia
Abstract
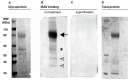
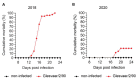
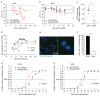
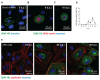
Infectious salmon anaemia virus (ISAV) binds circulating Atlantic salmon erythrocytes, but the relevance of this interaction for the course of infection and development of disease remains unclear. We here characterise ISAV-erythrocyte interactions in experimentally infected Atlantic salmon and show that ISAV-binding to erythrocytes is common and precedes the development of disease. Viral RNA and infective particles were enriched in the cellular fraction of blood. While erythrocyte-associated ISAV remained infectious, erythrocytes dose-dependently limited the infection of cultured cells. Surprisingly, immunostaining of blood smears revealed expression of ISAV proteins in a small fraction of erythrocytes in one of the examined trials, confirming that ISAV can be internalised in this cell type and engage the cellular machinery in transcription and translation. However, viral protein expression in erythrocytes was rare and not required for development of disease and mortality. Furthermore, active transcription of ISAV mRNA was higher in tissues than in blood, supporting the assumption that ISAV replication predominantly takes place in endothelial cells. In conclusion, Atlantic salmon erythrocytes bind ISAV and sequester infective virus particles during infection, but do not appear to significantly contribute to ISAV replication. We discuss the implications of our findings for infection dynamics and pathogenesis of infectious salmon anaemia.
Keywords: adsorption; decoy; isavirus; nucleated erythrocyte; orthomyxovirus; red blood cell; viral replication.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Infectious salmon anaemia virus replication and induction of alpha interferon in Atlantic salmon erythrocytes.Virol J. 2008 Feb 28;5:36. doi: 10.1186/1743-422X-5-36. Virol J. 2008. PMID: 18307775 Free PMC article.
-
Demonstration of infectious salmon anaemia virus (ISAV) endocytosis in erythrocytes of Atlantic salmon.Virol J. 2007 Jan 25;4:13. doi: 10.1186/1743-422X-4-13. Virol J. 2007. PMID: 17254352 Free PMC article.
-
First detection, isolation and molecular characterization of infectious salmon anaemia virus associated with clinical disease in farmed Atlantic salmon (Salmo salar) in Chile.BMC Vet Res. 2008 Aug 4;4:28. doi: 10.1186/1746-6148-4-28. BMC Vet Res. 2008. PMID: 18680586 Free PMC article.
-
Infectious salmon anaemia - pathogenesis and tropism.J Fish Dis. 2014 Apr;37(4):291-307. doi: 10.1111/jfd.12225. Epub 2014 Jan 30. J Fish Dis. 2014. PMID: 24475971 Review.
-
Infectious salmon anaemia virus-molecular biology and pathogenesis of the infection.J Appl Microbiol. 2020 Jul;129(1):85-97. doi: 10.1111/jam.14567. Epub 2020 Jan 16. J Appl Microbiol. 2020. PMID: 31885186 Review.
Cited by
-
Long-term persistence of piscine orthoreovirus-1 (PRV-1) infection during the pre-smolt stages of Atlantic salmon in freshwater.Vet Res. 2023 Aug 29;54(1):69. doi: 10.1186/s13567-023-01201-w. Vet Res. 2023. PMID: 37644605 Free PMC article.
-
Destruction of the vascular viral receptor in infectious salmon anaemia provides in vivo evidence of homologous attachment interference.PLoS Pathog. 2022 Oct 14;18(10):e1010905. doi: 10.1371/journal.ppat.1010905. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36240255 Free PMC article.
References
-
- Christiansen D.H., McBeath A.J.A., Aamelfot M., Matejusova I., Fourrier M., White P., Petersen P.E., Falk K. First field evidence of the evolution from a non-virulent HPR0 to a virulent HPR-deleted infectious salmon anaemia virus. J. Gen. Virol. 2017;98:595–606. doi: 10.1099/jgv.0.000741. - DOI - PubMed
-
- Aquatic Animal Health Code. [(accessed on 31 August 2021)]. Available online: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/aquatic-co...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

