Visualization of Retroviral Gag-Genomic RNA Cellular Interactions Leading to Genome Encapsidation and Viral Assembly: An Overview
- PMID: 35215917
- PMCID: PMC8876502
- DOI: 10.3390/v14020324
Visualization of Retroviral Gag-Genomic RNA Cellular Interactions Leading to Genome Encapsidation and Viral Assembly: An Overview
Abstract
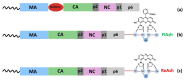
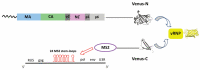
Retroviruses must selectively recognize their unspliced RNA genome (gRNA) among abundant cellular and spliced viral RNAs to assemble into newly formed viral particles. Retroviral gRNA packaging is governed by Gag precursors that also orchestrate all the aspects of viral assembly. Retroviral life cycles, and especially the HIV-1 one, have been previously extensively analyzed by several methods, most of them based on molecular biology and biochemistry approaches. Despite these efforts, the spatio-temporal mechanisms leading to gRNA packaging and viral assembly are only partially understood. Nevertheless, in these last decades, progress in novel bioimaging microscopic approaches (as FFS, FRAP, TIRF, and wide-field microscopy) have allowed for the tracking of retroviral Gag and gRNA in living cells, thus providing important insights at high spatial and temporal resolution of the events regulating the late phases of the retroviral life cycle. Here, the implementation of these recent bioimaging tools based on highly performing strategies to label fluorescent macromolecules is described. This report also summarizes recent gains in the current understanding of the mechanisms employed by retroviral Gag polyproteins to regulate molecular mechanisms enabling gRNA packaging and the formation of retroviral particles, highlighting variations and similarities among the different retroviruses.
Keywords: Gag precursor; cellular trafficking; fluorescent labelling; genomic RNA packaging; live-cells microscopy; plasma membrane; retroviral assembly; retrovirus; ribonucleoprotein complex; specific interactions.
Conflict of interest statement
No conflict of interest declared.
Figures

References
-
- Lever A.M.L. Advances in Pharmacology. Volume 48. Elsevier; Amsterdam, The Netherlands: 2000. HIV RNA Packaging and Lentivirus-Based Vectors; pp. 1–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

