High-Throughput Platform for Detection of Neutralizing Antibodies Using Flavivirus Reporter Replicon Particles
- PMID: 35215941
- PMCID: PMC8880525
- DOI: 10.3390/v14020346
High-Throughput Platform for Detection of Neutralizing Antibodies Using Flavivirus Reporter Replicon Particles
Abstract
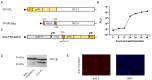
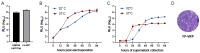
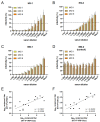
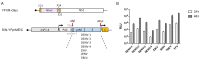
Flavivirus outbreaks require fast and reliable diagnostics that can be easily adapted to newly emerging and re-emerging flaviviruses. Due to the serological cross-reactivity among flavivirus antibodies, neutralization tests (NT) are considered the gold standard for sero-diagnostics. Here, we first established wild-type single-round infectious virus replicon particles (VRPs) by packaging a yellow fever virus (YFV) replicon expressing Gaussia luciferase (Gluc) with YFV structural proteins in trans using a double subgenomic Sindbis virus (SINV) replicon. The latter expressed the YFV envelope proteins prME via the first SINV subgenomic promoter and the capsid protein via a second subgenomic SINV promoter. VRPs were produced upon co-electroporation of replicon and packaging RNA. Introduction of single restriction enzyme sites in the packaging construct flanking the prME sequence easily allowed to exchange the prME moiety resulting in chimeric VRPs that have the surface proteins of other flaviviruses including dengue virus 1--4, Zika virus, West Nile virus, and tick-borne encephalitis virus. Besides comparing the YF-VRP based NT assay to a YF reporter virus NT assay, we analyzed the neutralization efficiencies of different human anti-flavivirus sera or a monoclonal antibody against all established VRPs. The assays were performed in a 96-well high-throughput format setting with Gluc as readout in comparison to classical plaque reduction NTs indicating that the VRP-based NT assays are suitable for high-throughput analyses of neutralizing flavivirus antibodies.
Keywords: diagnostics; flavivirus; high-throughput; neutralization assay; virus replicon particles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- de Oliveira Figueiredo P., Stoffella-Dutra A.G., Barbosa Costa G., Silva de Oliveira J., Dourado Amaral C., Duarte Santos J., Soares Rocha K.L., Araújo Júnior J.P., Lacerda Nogueira M., Zazá Borges M.A., et al. Re-Emergence of Yellow Fever in Brazil during 2016–2019: Challenges, Lessons Learned, and Perspectives. Viruses. 2020;12:1233. doi: 10.3390/v12111233. - DOI - PMC - PubMed
-
- WHO Yellow Fever—Brazil. [(accessed on 22 December 2021)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/11-february-2....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

