Encephalomyocarditis Virus 2A Protein Inhibited Apoptosis by Interaction with Annexin A2 through JNK/c-Jun Pathway
- PMID: 35215950
- PMCID: PMC8880565
- DOI: 10.3390/v14020359
Encephalomyocarditis Virus 2A Protein Inhibited Apoptosis by Interaction with Annexin A2 through JNK/c-Jun Pathway
Abstract
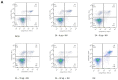
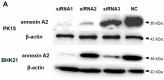
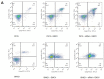
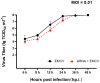
Encephalomyocarditis virus can cause myocarditis and encephalitis in pigs and other mammals, thus posing a potential threat to public health safety. The 2A protein is an important virulence factor of EMCV. Previous studies have shown that the 2A protein may be related to the inhibition of apoptosis by virus, but its specific molecular mechanism is not clear. In this study, the 2A protein was expressed in Escherichia coli in order to find interacting cell proteins. A pull down assay, coupled with mass spectrometry, revealed that the 2A protein possibly interacted with annexin A2. Co-immunoprecipitation assays and confocal imaging analysis further demonstrated that the 2A protein interacted with annexin A2 in cells. In reducing the expression of annexin A2 by siRNA, the ability of the 2A protein to inhibit apoptosis was weakened and the proliferation of EMCV was slowed down. These results suggest that annexin A2 is closely related to the inhibition of apoptosis by 2A. Furthermore, both RT-PCR and western blot results showed that the 2A protein requires annexin A2 interaction to inhibit apoptosis via JNK/c-Jun pathway. Taken together, our data indicate that the 2A protein inhibits apoptosis by interacting with annexin A2 via the JNK/c-Jun pathway. These findings provide insight into the molecular pathogenesis underlying EMCV infection.
Keywords: 2A; JNK/c-Jun pathway; annexin A2; apoptosis; encephalomyocarditis virus.
Conflict of interest statement
The author declares that there are no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

