The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir
- PMID: 35215955
- PMCID: PMC8880184
- DOI: 10.3390/v14020362
The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir
Abstract
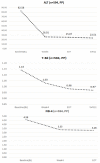
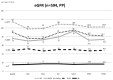
Background: The results of long-term renal evolution in HCV-infected patients using sofosbuvir and velpatasvir (SOF/VEL), with or without ribavirin (RBV), are lacking. Aims: We evaluated the renal safety for HCV-infected patients receiving SOF/VEL. Methods: Between 1 June 2019 and 6 July 2020, we included 594 HCV-infected patients receiving SOF/VEL +/- RBV for 12 weeks in Taiwan. Viral eradication rate (defined by sustained virological response at week 12 post-treatment; SVR12) and changes to renal function were considered. Results: SVR12 was achieved in 99.3% (590/594) upon per-protocol analysis. Patients saw improved hepatobiliary function and fibrosis after the start of SOF/VEL therapy. For renal function, those with baseline estimated glomerular filtration rate (eGFR) ≥ 60 (mL/min/1.73 m2) experienced transient on-treatment reduction in renal function that improved upon ending treatment, but recurrent eGFR degradation during one-year follow-up. The use of RBV (OR = 5.200, 95% CI: 1.983-13.634, p = 0.001) was a significant risk factor at SVR24, while diabetes mellitus (OR = 2.765, 95% CI: 1.104-6.922, p = 0.030) and the use of RBV (OR = 3.143, 95% CI: 1.047-9.435, p = 0.041) were identified as significant risk factors of worsening renal function at SVR48. SOF/VEL did not worsen renal function among those with stage 4-5 chronic kidney disease (CKD) who were not receiving dialysis. Conclusions: A trend of decline in eGFR at 1 year after SOF/VEL treatment was observed among diabetic patients with baseline eGFR ≥ 60 (mL/min/1.73 m2) and concomitant use of RBV. The close monitoring of renal function is warranted. Further study should be conducted in order to weigh the risks and benefit of RBV.
Keywords: direct-acting antivirals; hepatitis C virus; renal function; sofosbuvir and velpatasvir.
Conflict of interest statement
The authors declare no conflict of interest related to the study.
Figures
Similar articles
-
Sofosbuvir/velpatasvir with or without low-dose ribavirin for patients with chronic hepatitis C virus infection and severe renal impairment.Gut. 2022 Jan;71(1):176-184. doi: 10.1136/gutjnl-2020-323569. Epub 2021 Jan 6. Gut. 2022. PMID: 33408122
-
Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3.J Hepatol. 2019 Jan;70(1):15-23. doi: 10.1016/j.jhep.2018.09.018. Epub 2018 Sep 26. J Hepatol. 2019. PMID: 30266283
-
Effectiveness and safety of sofosbuvir/velpatasvir ± ribavirin vs glecaprevir/pibrentasvir in genotype 3 hepatitis C virus infected patients.Eur J Hosp Pharm. 2020 Mar;27(e1):e41-e47. doi: 10.1136/ejhpharm-2019-002060. Epub 2020 Feb 7. Eur J Hosp Pharm. 2020. PMID: 32296504 Free PMC article.
-
Real-World Effectiveness of Direct-Acting Antiviral Regimens against Hepatitis C Virus (HCV) Genotype 3 Infection: A Systematic Review and Meta-Analysis.Ann Hepatol. 2021 Jul-Aug;23:100268. doi: 10.1016/j.aohep.2020.09.012. Epub 2020 Oct 12. Ann Hepatol. 2021. PMID: 33059055
-
Sofosbuvir/Velpatasvir: The First Pangenotypic Direct-Acting Antiviral Combination for Hepatitis C.Ann Pharmacother. 2017 Jan;51(1):44-53. doi: 10.1177/1060028016668897. Epub 2016 Oct 1. Ann Pharmacother. 2017. PMID: 27609942 Review.
Cited by
-
Factors Associated with Large Renal Function Decline in Patients with Chronic Hepatitis C Successfully Treated with Direct-Acting Antiviral Therapy.Diagnostics (Basel). 2023 Jan 27;13(3):473. doi: 10.3390/diagnostics13030473. Diagnostics (Basel). 2023. PMID: 36766578 Free PMC article.
References
-
- Jordan J.F., Ira M.J., Hézode C., Asselah T., Ruane P.J., Gruener N., Abergel A., Mangia A., Lai C.-L., Chan H.L.Y., et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 2015;373:2599–2607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous