Vav Proteins in Development of the Brain: A Potential Relationship to the Pathogenesis of Congenital Zika Syndrome?
- PMID: 35215978
- PMCID: PMC8874935
- DOI: 10.3390/v14020386
Vav Proteins in Development of the Brain: A Potential Relationship to the Pathogenesis of Congenital Zika Syndrome?
Abstract
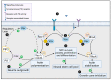
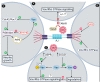
Zika virus (ZIKV) infection during pregnancy can result in a significant impact on the brain and eye of the developing fetus, termed congenital zika syndrome (CZS). At a morphological level, the main serious presentations of CZS are microcephaly and retinal scarring. At a cellular level, many cell types of the brain may be involved, but primarily neuronal progenitor cells (NPC) and developing neurons. Vav proteins have guanine exchange activity in converting GDP to GTP on proteins such as Rac1, Cdc42 and RhoA to stimulate intracellular signaling pathways. These signaling pathways are known to play important roles in maintaining the polarity and self-renewal of NPC pools by coordinating the formation of adherens junctions with cytoskeletal rearrangements. In developing neurons, these same pathways are adopted to control the formation and growth of neurites and mediate axonal guidance and targeting in the brain and retina. This review describes the role of Vavs in these processes and highlights the points of potential ZIKV interaction, such as (i) the binding and entry of ZIKV in cells via TAM receptors, which may activate Vav/Rac/RhoA signaling; (ii) the functional convergence of ZIKV NS2A with Vav in modulating adherens junctions; (iii) ZIKV NS4A/4B protein effects on PI3K/AKT in a regulatory loop via PPI3 to influence Vav/Rac1 signaling in neurite outgrowth; and (iv) the induction of SOCS1 and USP9X following ZIKV infection to regulate Vav protein degradation or activation, respectively, and impact Vav/Rac/RhoA signaling in NPC and neurons. Experiments to define these interactions will further our understanding of the molecular basis of CZS and potentially other developmental disorders stemming from in utero infections. Additionally, Vav/Rac/RhoA signaling pathways may present tractable targets for therapeutic intervention or molecular rationale for disease severity in CZS.
Keywords: Rac1; RhoA; Vav; brain development; congenital zika syndrome; guanosine nucleotide exchange factor; neuronal development; neuronal progenitor cell; zika virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Petersen E., Wilson M.E., Touch S., McCloskey B., Mwaba P., Bates M., Dar O., Mattes F., Kidd M., Ippolito G., et al. Rapid Spread of Zika Virus in The Americas—Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int. J. Infect. Dis. 2016;44:11–15. doi: 10.1016/j.ijid.2016.02.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

