mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases
- PMID: 35215994
- PMCID: PMC8877136
- DOI: 10.3390/v14020401
mRNA Vaccine Development for Emerging Animal and Zoonotic Diseases
Abstract
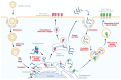
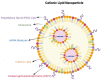
In the prevention and treatment of infectious diseases, mRNA vaccines hold great promise because of their low risk of insertional mutagenesis, high potency, accelerated development cycles, and potential for low-cost manufacture. In past years, several mRNA vaccines have entered clinical trials and have shown promise for offering solutions to combat emerging and re-emerging infectious diseases such as rabies, Zika, and influenza. Recently, the successful application of mRNA vaccines against COVID-19 has further validated the platform and opened the floodgates to mRNA vaccine's potential in infectious disease prevention, especially in the veterinary field. In this review, we describe our current understanding of the mRNA vaccines and the technologies used for mRNA vaccine development. We also provide an overview of mRNA vaccines developed for animal infectious diseases and discuss directions and challenges for the future applications of this promising vaccine platform in the veterinary field.
Keywords: immune response; infectious disease; mRNA vaccine; viruses; zoonoses.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
mRNA Vaccine Development in the Fight Against Zoonotic Viral Diseases.Viruses. 2025 Jul 8;17(7):960. doi: 10.3390/v17070960. Viruses. 2025. PMID: 40733577 Free PMC article. Review.
-
The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article.
-
mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases.Mol Ther. 2019 Apr 10;27(4):757-772. doi: 10.1016/j.ymthe.2019.01.020. Epub 2019 Feb 7. Mol Ther. 2019. PMID: 30803823 Free PMC article. Review.
-
mRNA vaccines for infectious diseases: principles, delivery and clinical translation.Nat Rev Drug Discov. 2021 Nov;20(11):817-838. doi: 10.1038/s41573-021-00283-5. Epub 2021 Aug 25. Nat Rev Drug Discov. 2021. PMID: 34433919 Free PMC article. Review.
-
From Sequence to System: Enhancing IVT mRNA Vaccine Effectiveness through Cutting-Edge Technologies.Mol Pharm. 2025 Jan 6;22(1):81-102. doi: 10.1021/acs.molpharmaceut.4c00863. Epub 2024 Nov 27. Mol Pharm. 2025. PMID: 39601789 Review.
Cited by
-
Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis.Front Immunol. 2023 Apr 14;14:1161507. doi: 10.3389/fimmu.2023.1161507. eCollection 2023. Front Immunol. 2023. PMID: 37122740 Free PMC article.
-
Immunocyte membrane-derived biomimetic nano-drug delivery system: a pioneering platform for tumour immunotherapy.Acta Pharmacol Sin. 2024 Dec;45(12):2455-2473. doi: 10.1038/s41401-024-01355-z. Epub 2024 Jul 31. Acta Pharmacol Sin. 2024. PMID: 39085407 Review.
-
Infection and Prevention of Rabies Viruses.Microorganisms. 2025 Feb 9;13(2):380. doi: 10.3390/microorganisms13020380. Microorganisms. 2025. PMID: 40005749 Free PMC article. Review.
-
Innovation in mRNA Vaccines and RNAi via Protein Nanocages.Vaccines (Basel). 2025 Jun 18;13(6):653. doi: 10.3390/vaccines13060653. Vaccines (Basel). 2025. PMID: 40573984 Free PMC article. Review.
-
Virulence and Immune Evasion Strategies of FMDV: Implications for Vaccine Design.Vaccines (Basel). 2024 Sep 19;12(9):1071. doi: 10.3390/vaccines12091071. Vaccines (Basel). 2024. PMID: 39340101 Free PMC article. Review.
References
-
- Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., et al. Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Healthy Adults Aged 18-59 Years: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. - DOI - PMC - PubMed
-
- al Kaabi N., Zhang Y., Xia S., Yang Y., al Qahtani M.M., Abdulrazzaq N., Al Nusair M., Hassany M., Jawad J.S., Abdalla J., et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

