Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio
- PMID: 35216005
- PMCID: PMC8876716
- DOI: 10.3390/v14020412
Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio
Abstract
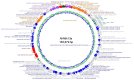
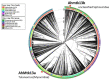
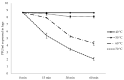
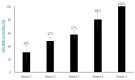
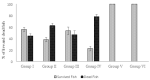
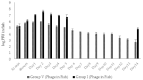
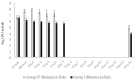
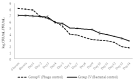
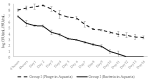
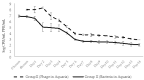
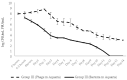
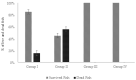
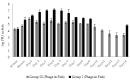
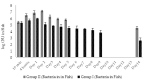
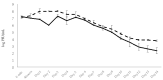
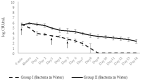
Phage therapy can be an effective alternative to standard antimicrobial chemotherapy for control of Aeromonas hydrophila infections in aquaculture. Aeromonas hydrophila-specific phages AhMtk13a and AhMtk13b were studied for basic biological properties and genome characteristics. Phage AhMtk13a (Myovirus, 163,879 bp genome, 41.21% CG content) was selected based on broad lytic spectrum and physiologic parameters indicating its lytic nature. The therapeutic potential of phage AhMtk13a was evaluated in experimental studies in zebrafish challenged with A. hydrophila GW3-10 via intraperitoneal injection and passive immersion in aquaria water. In experimental series 1 with single introduction of AhMtk13a phage to aquaria water at phage-bacteria ratio 10:1, cumulative mortality 44% and 62% was registered in fish exposed to phage immediately and in 4 h after bacterial challenge, correspondingly, compared to 78% mortality in the group with no added phage. In experimental series 2 with triple application of AhMtk13a phage at ratio 100:1, the mortality comprised 15% in phage-treated group compared to the 55% in the control group. Aeromonas hydrophila GW3-10 was not detectable in aquaria water from day 9 but still present in fish at low concentration. AhMtk13a phage was maintained in fish and water throughout the experiment at the higher concentration in infected fish.
Keywords: Aeromonas hydrophila; aquaculture; bacteriophage; phage genome; phage therapy; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- FAO . The State of World Fisheries and Aquaculture. FAO; Rome, Italy: 2018. Meeting the Sustainable Development Goals.
-
- EUGeorgia Fisheries and Aquaculture in Georgia (Industry Research) 2020. [(accessed on 13 December 2021)]. Available online: http://eugeorgia.info/en/article/832/fisheries-and-aquaculture-in-georgi....
-
- Aly D.S.M. A Review of Fish Diseases in the Egyptian Aquaculture Sector. CGIAR; Montpellier, France: 2013. Working Report. 41.
-
- Chandrarathna H.P.S.U., Nikapitiya C., Dananjaya S.H.S., De Silva B.C.J., Heo G.-J., De Zoysa M., Lee J. Isolation and Characterization of Phage AHP-1 and Its Combined Effect with Chloramphenicol to Control, Aeromonas hydrophila. Braz. J. Microbiol. 2020;51:409–416. doi: 10.1007/s42770-019-00178-z. - DOI - PMC - PubMed
-
- Chandrarathna H.P.S.U., Nikapitiya C., Dananjaya S.H.S., Wijerathne C.U.B., Wimalasena S.H.M.P., Kwun H.J., Heo G.-J., Lee J., De Zoysa M. Outcome of Co-Infection with Opportunistic and Multidrug Resistant Aeromonas hydrophila and A. veronii in Zebrafish: Identification, Characterization, Pathogenicity and Immune Responses. Fish Shellfish Immunol. 2018;80:573–581. doi: 10.1016/j.fsi.2018.06.049. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

