Transkingdom Analysis of the Female Reproductive Tract Reveals Bacteriophages form Communities
- PMID: 35216023
- PMCID: PMC8878565
- DOI: 10.3390/v14020430
Transkingdom Analysis of the Female Reproductive Tract Reveals Bacteriophages form Communities
Abstract
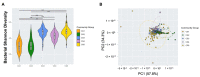
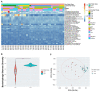
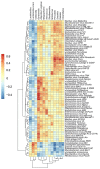
The female reproductive tract (FRT) microbiome plays a vital role in maintaining vaginal health. Viruses are key regulators of other microbial ecosystems, but little is known about how the FRT viruses (virome), particularly bacteriophages that comprise the phageome, impact FRT health and dysbiosis. We hypothesize that bacterial vaginosis (BV) is associated with altered FRT phageome diversity, transkingdom interplay, and bacteriophage discriminate taxa. Here, we conducted a retrospective, longitudinal analysis of vaginal swabs collected from 54 BV-positive and 46 BV-negative South African women. Bacteriome analysis revealed samples clustered into five distinct bacterial community groups (CGs), and further, bacterial alpha diversity was significantly associated with BV. Virome analysis on a subset of baseline samples showed FRT bacteriophages clustering into novel viral state types (VSTs), a viral community clustering system based on virome composition and abundance. Distinct BV bacteriophage signatures included increased alpha diversity along with discriminant Bacillus, Burkholderia, and Escherichia bacteriophages. Bacteriophage-bacteria transkingdom associations were also identified between Bacillus and Burkholderia viruses and BV-associated bacteria, providing key insights for future studies elucidating the transkingdom interactions driving BV-associated microbiome perturbations. In this cohort, bacteriophage-bacterial associations suggest complex interactions, which may play a role in the establishment and maintenance of BV.
Keywords: HIV; bacterial vaginosis; bacteriophage; female reproductive tract; microbiome; transkingdom associations; virome.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Gosmann C., Anahtar M.N., Handley S.A., Farcasanu M., Abu-Ali G., Bowman B.A., Padavattan N., Desai C., Droit L., Moodley A., et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity. 2017;46:29–37. doi: 10.1016/j.immuni.2016.12.013. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

