HOFs Built from Hexatopic Carboxylic Acids: Structure, Porosity, Stability, and Photophysics
- PMID: 35216044
- PMCID: PMC8875020
- DOI: 10.3390/ijms23041929
HOFs Built from Hexatopic Carboxylic Acids: Structure, Porosity, Stability, and Photophysics
Abstract
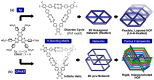
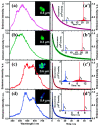
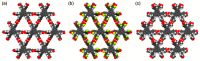
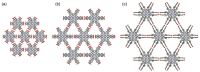
Hydrogen-bonded organic frameworks (HOFs) have attracted renewed attention as another type of promising candidates for functional porous materials. In most cases of HOF preparation, the applied molecular design principle is based on molecules with rigid π-conjugated skeleton together with more than three H-bonding groups to achieve 2D- or 3D-networked structures. However, the design principle does not always work, but results in formation of unexpected structures, where subtle structural factors of which we are not aware dictate the entire structure of HOFs. In this contribution, we assess recent advances in HOFs, focusing on those composed of hexatopic building block molecules, which can provide robust frameworks with a wide range of topologies and properties. The HOFs described in this work are classified into three types, depending on their H-bonded structural motifs. Here in, we focus on: (1) the chemical aspects that govern their unique fundamental chemistry and structures; and (2) their photophysics at the ensemble and single-crystal levels. The work addresses and discusses how these aspects affect and orient their photonic applicability. We trust that this contribution will provide a deep awareness and will help scientists to build up a systematic series of porous materials with the aim to control both their structural and photodynamical assets.
Keywords: hexatopic carboxylic acids; hydrogen-bonded organic frameworks; photophysics; porosity; stability; time-resolved spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

