Alpha 1 Antitrypsin Regulates Trophoblast Syncytialization and Inflammatory Factor Expression
- PMID: 35216073
- PMCID: PMC8879717
- DOI: 10.3390/ijms23041955
Alpha 1 Antitrypsin Regulates Trophoblast Syncytialization and Inflammatory Factor Expression
Abstract
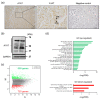
The serine protease inhibitor alpha1-antitrypsin (A1AT) may possess protective functions of impaired organs in a manner independent of its protease inhibitor activity. A1AT expression has been shown to fluctuate in patients with pregnancy-induced hypertension, which suggests that A1AT may play a role in the syncytialization of villous trophoblasts. A1AT expression was knocked down in primary trophoblasts. RNA was extracted from these cells and subjected to RNA-sequencing analysis to determine the levels of expression of markers of syncytialization and inflammation. In addition, A1AT protein was localized in trophoblastic cells in placental tissues. Knockdown of A1AT upregulated the expression of FOSL1 and markers of syncytialization, as well as cell fusion, whereas overexpression of A1AT had the opposite effects. FOSL1 overexpression stimulated syncytialization, similar to the effects of A1AT knock down. Inhibitors of p38MAPK and JNK reduce the expression of inflammatory factors, whereas a p38MAPK inhibitor suppressed FOSL1 expression. Collectively, these findings indicated A1AT may negatively regulate inflammatory responses by controlling the activation of p38MAPK and JNK, and that p38MAPK mediates trophoblast syncytialization by altering FOSL1 expression. Therefore, a dysfunction in A1AT could be responsible for abnormal placental formation and pregnancy-associated disorders.
Keywords: AP-1 transcription factor subunit (FOSL1); FOS like 1; alpha-1-antitrypsin (A1AT); inflammatory factor; syncytialization; trophoblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ando Y., Kuroda A., Kusama K., Matsutani T., Matsuda A., Tamura K. Impact of serine protease inhibitor alpha1-antitrypsin on expression of endoplasmic reticulum stress-induced proinflammatory factors in adipocytes. Biochem. Biophys. Rep. 2021;26:100967. doi: 10.1016/j.bbrep.2021.100967. - DOI - PMC - PubMed
-
- Jonigk D., Al-Omari M., Maegel L., Müller M., Izykowski N., Hong J., Hong K., Kim S.H., Dorsch M., Mahadeva R., et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA. 2013;110:15007–15012. doi: 10.1073/pnas.1309648110. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

