Lysophosphatidic Acid Promotes the Expansion of Cancer Stem Cells via TRPC3 Channels in Triple-Negative Breast Cancer
- PMID: 35216080
- PMCID: PMC8877950
- DOI: 10.3390/ijms23041967
Lysophosphatidic Acid Promotes the Expansion of Cancer Stem Cells via TRPC3 Channels in Triple-Negative Breast Cancer
Abstract
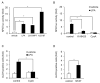
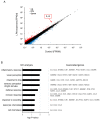
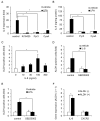
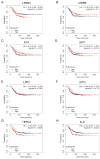
Triple-negative breast cancer (TNBC) is a highly aggressive cancer for which targeted therapeutic agents are limited. Growing evidence suggests that TNBC originates from breast cancer stem cells (BCSCs), and elucidation of the molecular mechanisms controlling BCSC proliferation will be crucial for new drug development. We have previously reported that the lysosphingolipid sphingosine-1-phosphate mediates the CSC phenotype, which can be identified as the ALDH-positive cell population in several types of human cancer cell lines. In this study, we have investigated additional lipid receptors upregulated in BCSCs. We found that lysophosphatidic acid (LPA) receptor 3 was highly expressed in ALDH-positive TNBC cells. The LPAR3 antagonist inhibited the increase in ALDH-positive cells after LPA treatment. Mechanistically, the LPA-induced increase in ALDH-positive cells was dependent on intracellular calcium ion (Ca2+), and the increase in Ca2+ was suppressed by a selective inhibitor of transient receptor potential cation channel subfamily C member 3 (TRPC3). Moreover, IL-8 production was involved in the LPA response via the activation of the Ca2+-dependent transcriptional factor nuclear factor of activated T cells. Taken together, our findings provide new insights into the lipid-mediated regulation of BCSCs via the LPA-TRPC3 signaling axis and suggest several potential therapeutic targets for TNBC.
Keywords: cancer stem cells; lysophosphatidic acid; nuclear factor of activated T cells; transient receptor potential cation channel subfamily C member 3; triple-negative breast cancer.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures






Similar articles
-
ADSCs and adipocytes are the main producers in the autotaxin-lysophosphatidic acid axis of breast cancer and healthy mammary tissue in vitro.BMC Cancer. 2018 Dec 19;18(1):1273. doi: 10.1186/s12885-018-5166-z. BMC Cancer. 2018. PMID: 30567518 Free PMC article.
-
TRPC3-mediated NFATc1 calcium signaling promotes triple negative breast cancer migration through regulating glypican-6 and focal adhesion.Pflugers Arch. 2025 Feb;477(2):253-272. doi: 10.1007/s00424-024-03030-y. Epub 2024 Oct 22. Pflugers Arch. 2025. PMID: 39436410 Free PMC article.
-
NOTCH4 maintains quiescent mesenchymal-like breast cancer stem cells via transcriptionally activating SLUG and GAS1 in triple-negative breast cancer.Theranostics. 2020 Jan 19;10(5):2405-2421. doi: 10.7150/thno.38875. eCollection 2020. Theranostics. 2020. PMID: 32104513 Free PMC article.
-
Cell surface receptors in lysophospholipid signaling.Semin Cell Dev Biol. 2004 Oct;15(5):457-65. doi: 10.1016/j.semcdb.2004.05.005. Semin Cell Dev Biol. 2004. PMID: 15271291 Review.
-
The hedgehog pathway in triple-negative breast cancer.Cancer Med. 2016 Oct;5(10):2989-3006. doi: 10.1002/cam4.833. Epub 2016 Aug 18. Cancer Med. 2016. PMID: 27539549 Free PMC article. Review.
Cited by
-
TRP Channels as Molecular Targets to Relieve Endocrine-Related Diseases.Front Mol Biosci. 2022 Apr 28;9:895814. doi: 10.3389/fmolb.2022.895814. eCollection 2022. Front Mol Biosci. 2022. PMID: 35573736 Free PMC article. Review.
-
Orai1 and Orai3 act through distinct signalling axes to promote stemness and tumorigenicity of breast cancer stem cells.Stem Cell Res Ther. 2024 Aug 13;15(1):256. doi: 10.1186/s13287-024-03875-1. Stem Cell Res Ther. 2024. PMID: 39135143 Free PMC article.
-
Crosstalk between Ca2+ Signaling and Cancer Stemness: The Link to Cisplatin Resistance.Int J Mol Sci. 2022 Sep 14;23(18):10687. doi: 10.3390/ijms231810687. Int J Mol Sci. 2022. PMID: 36142596 Free PMC article. Review.
-
Discovery of a potent and selective TRPC3 antagonist with neuroprotective effects.Bioorg Med Chem. 2025 Jan 1;117:118021. doi: 10.1016/j.bmc.2024.118021. Epub 2024 Nov 26. Bioorg Med Chem. 2025. PMID: 39612770
-
Calcium channels as pharmacological targets for cancer therapy.Clin Exp Med. 2025 Mar 25;25(1):94. doi: 10.1007/s10238-025-01632-z. Clin Exp Med. 2025. PMID: 40131496 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

