Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy
- PMID: 35216097
- PMCID: PMC8875744
- DOI: 10.3390/ijms23041981
Microfluidic-Based Technologies for CTC Isolation: A Review of 10 Years of Intense Efforts towards Liquid Biopsy
Abstract
The selection of circulating tumor cells (CTCs) directly from blood as a real-time liquid biopsy has received increasing attention over the past ten years, and further analysis of these cells may greatly aid in both research and clinical applications. CTC analysis could advance understandings of metastatic cascade, tumor evolution, and patient heterogeneity, as well as drug resistance. Until now, the rarity and heterogeneity of CTCs have been technical challenges to their wider use in clinical studies, but microfluidic-based isolation technologies have emerged as promising tools to address these limitations. This review provides a detailed overview of latest and leading microfluidic devices implemented for CTC isolation. In particular, this study details must-have device performances and highlights the tradeoff between recovery and purity. Finally, the review gives a report of CTC potential clinical applications that can be conducted after CTC isolation. Widespread microfluidic devices, which aim to support liquid-biopsy-based applications, will represent a paradigm shift for cancer clinical care in the near future.
Keywords: CTC isolation; circulating tumor cells; downstream analysis; liquid biopsy; microfluidic devices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




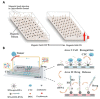


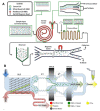
References
-
- Ashworth T.R. A Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. Med. J. Aust. 1869;14:146–147.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

