Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors
- PMID: 35216098
- PMCID: PMC8880247
- DOI: 10.3390/ijms23041982
Genome-Wide Expression and Anti-Proliferative Effects of Electric Field Therapy on Pediatric and Adult Brain Tumors
Abstract
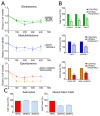
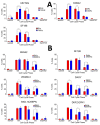
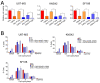
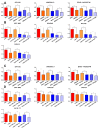
The lack of treatment options for high-grade brain tumors has led to searches for alternative therapeutic modalities. Electrical field therapy is one such area. The Optune™ system is an FDA-approved novel device that delivers continuous alternating electric fields (tumor treating fields-TTFields) to the patient for the treatment of primary and recurrent Glioblastoma multiforme (GBM). Various mechanisms have been proposed to explain the effects of TTFields and other electrical therapies. Here, we present the first study of genome-wide expression of electrotherapy (delivered via TTFields or Deep Brain Stimulation (DBS)) on brain tumor cell lines. The effects of electric fields were assessed through gene expression arrays and combinational effects with chemotherapies. We observed that both DBS and TTFields significantly affected brain tumor cell line viability, with DBS promoting G0-phase accumulation and TTFields promoting G2-phase accumulation. Both treatments may be used to augment the efficacy of chemotherapy in vitro. Genome-wide expression assessment demonstrated significant overlap between the different electrical treatments, suggesting novel interactions with mitochondrial functioning and promoting endoplasmic reticulum stress. We demonstrate the in vitro efficacy of electric fields against adult and pediatric high-grade brain tumors and elucidate potential mechanisms of action for future study.
Keywords: Deep Brain Stimulation; ependymoma; glioma; tumor treating fields.
Conflict of interest statement
The funders and Novocure had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Stupp R., Wong E.T., Kanner A.A., Steinberg D., Engelhard H., Heidecke V., Kirson E.D., Taillibert S., Liebermann F., Dbalý V., et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D.M., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma. JAMA. 2017;318:2306–2311. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

