New Insights on the Nuclear Functions and Targeting of FAK in Cancer
- PMID: 35216114
- PMCID: PMC8874710
- DOI: 10.3390/ijms23041998
New Insights on the Nuclear Functions and Targeting of FAK in Cancer
Abstract
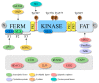
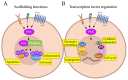
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase over-expressed and activated in both adult and pediatric cancers, where it plays important roles in the regulation of pathogenesis and progression of the malignant phenotype. FAK exerts its functions in cancer by two different ways: a kinase activity in the cytoplasm, mainly dependent on the integrin signaling, and a scaffolding activity into the nucleus by networking with different gene expression regulators. For this reason, FAK has to be considered a target with high therapeutic values. Indeed, evidence suggests that FAK targeting could be effective, either alone or in combination, with other already available treatments. Here, we propose an overview of the novel insights about FAK's structure and nuclear functions, with a special focus on the recent findings concerning the roles of this protein in cancer. Additionally, we provide a recent update on FAK inhibitors that are currently in clinical trials for patients with cancer, and discuss the challenge and future directions of drug-based anti-FAK targeted therapies.
Keywords: ATP-competitive inhibitors; FAK; PROTACs; adult cancers; allosteric inhibitors; combination therapy; pediatric cancers; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The roles of nuclear focal adhesion kinase (FAK) on Cancer: a focused review.J Exp Clin Cancer Res. 2019 Jun 11;38(1):250. doi: 10.1186/s13046-019-1265-1. J Exp Clin Cancer Res. 2019. PMID: 31186061 Free PMC article. Review.
-
Targeting focal adhesion kinase (FAK) for cancer therapy: FAK inhibitors, FAK-based dual-target inhibitors and PROTAC degraders.Biochem Pharmacol. 2024 Jun;224:116246. doi: 10.1016/j.bcp.2024.116246. Epub 2024 Apr 27. Biochem Pharmacol. 2024. PMID: 38685282 Review.
-
Focal adhesion kinase signaling - tumor vulnerabilities and clinical opportunities.J Cell Sci. 2024 Jul 15;137(14):jcs261723. doi: 10.1242/jcs.261723. Epub 2024 Jul 22. J Cell Sci. 2024. PMID: 39034922 Free PMC article. Review.
-
Development of focal adhesion kinase inhibitors in cancer therapy.Anticancer Agents Med Chem. 2011 Sep;11(7):638-42. doi: 10.2174/187152011796817628. Anticancer Agents Med Chem. 2011. PMID: 21787276 Review.
-
Identification of novel focal adhesion kinase substrates: role for FAK in NFκB signaling.Int J Biol Sci. 2015 Feb 17;11(4):404-10. doi: 10.7150/ijbs.10273. eCollection 2015. Int J Biol Sci. 2015. PMID: 25798060 Free PMC article.
Cited by
-
Differential response of patient-derived primary glioblastoma cells to metabolic and adhesion inhibitors.Clin Exp Med. 2025 Jun 26;25(1):220. doi: 10.1007/s10238-025-01736-6. Clin Exp Med. 2025. PMID: 40569470 Free PMC article.
-
Radioresistance in rhabdomyosarcomas: Much more than a question of dose.Front Oncol. 2022 Sep 29;12:1016894. doi: 10.3389/fonc.2022.1016894. eCollection 2022. Front Oncol. 2022. PMID: 36248991 Free PMC article. Review.
-
LAIR1 drives glioma progression by nuclear focal adhesion kinase dependent expressions of cyclin D1 and immunosuppressive chemokines/cytokines.Cell Death Dis. 2023 Oct 16;14(10):684. doi: 10.1038/s41419-023-06199-9. Cell Death Dis. 2023. PMID: 37845206 Free PMC article.
-
FERM domain-containing proteins are active components of the cell nucleus.Life Sci Alliance. 2024 Jan 31;7(4):e202302489. doi: 10.26508/lsa.202302489. Print 2024 Apr. Life Sci Alliance. 2024. PMID: 38296350 Free PMC article. Review.
-
A Novel In Vitro Model of the Bone Marrow Microenvironment in Acute Myeloid Leukemia Identifies CD44 and Focal Adhesion Kinase as Therapeutic Targets to Reverse Cell Adhesion-Mediated Drug Resistance.Cancers (Basel). 2025 Jan 3;17(1):135. doi: 10.3390/cancers17010135. Cancers (Basel). 2025. PMID: 39796762 Free PMC article.
References
-
- Seong J., Tajik A., Sun J., Guan J.-L., Humphries M.J., Craig S.E., Shekaran A., Garcia A.J., Lu S., Lin M.Z., et al. Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc. Natl. Acad. Sci. USA. 2013;110:19372–19377. doi: 10.1073/pnas.1307405110. - DOI - PMC - PubMed
-
- Romito I., Porru M., Braghini M.R., Pompili L., Panera N., Crudele A., Gnani D., De Stefanis C., Scarsella M., Pomella S., et al. Focal adhesion kinase inhibitor TAE226 combined with Sorafenib slows down hepatocellular carcinoma by multiple epigenetic effects. J. Exp. Clin. Cancer Res. 2021;40:364. doi: 10.1186/s13046-021-02154-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

