The Thermal Stability of the Collagen Triple Helix Is Tuned According to the Environmental Temperature
- PMID: 35216155
- PMCID: PMC8877210
- DOI: 10.3390/ijms23042040
The Thermal Stability of the Collagen Triple Helix Is Tuned According to the Environmental Temperature
Abstract
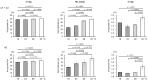
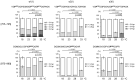
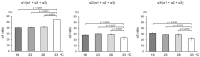
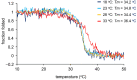
Triple helix formation of procollagen occurs in the endoplasmic reticulum (ER) where the single-stranded α-chains of procollagen undergo extensive post-translational modifications. The modifications include prolyl 4- and 3-hydroxylations, lysyl hydroxylation, and following glycosylations. The modifications, especially prolyl 4-hydroxylation, enhance the thermal stability of the procollagen triple helix. Procollagen molecules are transported to the Golgi and secreted from the cell, after the triple helix is formed in the ER. In this study, we investigated the relationship between the thermal stability of the collagen triple helix and environmental temperature. We analyzed the number of collagen post-translational modifications and thermal melting temperature and α-chain composition of secreted type I collagen in zebrafish embryonic fibroblasts (ZF4) cultured at various temperatures (18, 23, 28, and 33 °C). The results revealed that thermal stability and other properties of collagen were almost constant when ZF4 cells were cultured below 28 °C. By contrast, at a higher temperature (33 °C), an increase in the number of post-translational modifications and a change in α-chain composition of type I collagen were observed; hence, the collagen acquired higher thermal stability. The results indicate that the thermal stability of collagen could be autonomously tuned according to the environmental temperature in poikilotherms.
Keywords: collagen; environmental temperature; post-translational modification; thermal stability; triple helix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

