Selenium-Rich Yeast Peptide Fraction Ameliorates Imiquimod-Induced Psoriasis-like Dermatitis in Mice by Inhibiting Inflammation via MAPK and NF-κB Signaling Pathways
- PMID: 35216231
- PMCID: PMC8875820
- DOI: 10.3390/ijms23042112
Selenium-Rich Yeast Peptide Fraction Ameliorates Imiquimod-Induced Psoriasis-like Dermatitis in Mice by Inhibiting Inflammation via MAPK and NF-κB Signaling Pathways
Abstract
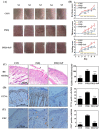
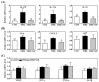
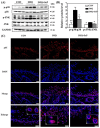
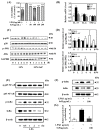
Psoriasis, a chronic and immune-mediated inflammatory disease, adversely affects patients' lives. We previously prepared selenium-rich yeast peptide fraction (SeP) from selenium-rich yeast protein hydrolysate and found that SeP could effectively alleviate ultraviolet radiation-induced skin damage in mice and inhibited H2O2-induced cytotoxicity in cultured human epidermal keratinocyte (HaCaT) cells. This study aimed to investigate whether SeP had a protective effect on imiquimod (IMQ)-induced psoriasis-like dermatitis in mice and the underlying mechanisms. Results showed that SeP significantly ameliorated the severity of skin lesion in IMQ-induced psoriasis-like mouse model. Moreover, SeP treatment significantly attenuated the expression of key inflammatory cytokines, including interleukin (IL)-23, IL-17A, and IL-17F, in the dorsal skin of mice. Mechanistically, SeP application not only inhibited the activation of JNK and p38 MAPK, but also the translocation of NF-κB into the nucleus in the dorsal skin. Furthermore, SeP treatment inhibited the levels of inflammatory cytokines and the activation of MAPK and NF-κB signaling induced by lipopolysaccharide in HaCaT cells and macrophage cell line RAW264.7. Overall, our findings showed that SeP alleviated psoriasis-like skin inflammation by inhibiting MAPK and NF-κB signaling pathways, which suggested that SeP would have a potential therapeutic effect against psoriasis.
Keywords: MAPK; NF-κB; inflammation; psoriasis; selenium-rich yeast peptide fraction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Global Report on Psoriasis. [(accessed on 26 October 2016)]. Available online: https://www.who.int/publications/i/item/global-report-on-psoriasis.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

