Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes
- PMID: 35216246
- PMCID: PMC8879503
- DOI: 10.3390/ijms23042129
Distinct Cold Acclimation of Productivity Traits in Arabidopsis thaliana Ecotypes
Abstract
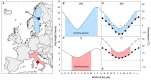
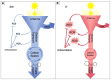
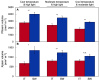
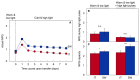
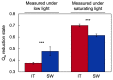
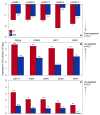
Improvement of crop climate resilience will require an understanding of whole-plant adaptation to specific local environments. This review places features of plant form and function related to photosynthetic productivity, as well as associated gene-expression patterns, into the context of the adaptation of Arabidopsis thaliana ecotypes to local environments with different climates in Sweden and Italy. The growth of plants under common cool conditions resulted in a proportionally greater emphasis on the maintenance of photosynthetic activity in the Swedish ecotype. This is compared to a greater emphasis on downregulation of light-harvesting antenna size and upregulation of a host of antioxidant enzymes in the Italian ecotype under these conditions. This differential response is discussed in the context of the climatic patterns of the ecotypes' native habitats with substantial opportunity for photosynthetic productivity under mild temperatures in Italy but not in Sweden. The Swedish ecotype's response is likened to pushing forward at full speed with productivity under low temperature versus the Italian ecotype's response of staying safe from harm (maintaining redox homeostasis) while letting productivity decline when temperatures are transiently cold. It is concluded that either strategy can offer directions for the development of climate-resilient crops for specific locations of cultivation.
Keywords: Lhcb; daylength; excitation pressure; freezing; growth; high light; nonphotochemical quenching; phloem; photosynthetic capacity; xylem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Intergovernmental Panel on Climate Change. [(accessed on 23 December 2021)]. Available online: http://www.ipcc.ch.
-
- Soriano M.A., Orgaz F., Villalobos F.J., Fereres E. Efficiency of water use of early plantings of sunflower. Eur. J. Agron. 2004;21:465–476. doi: 10.1016/j.eja.2004.07.001. - DOI
-
- Vitt P., Havens K., Kramer A.T., Sollenberger D., Yates E. Assisted migration of plants: Changes in latitudes, changes in attitudes. Biol. Conserv. 2010;143:18–27. doi: 10.1016/j.biocon.2009.08.015. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

