Cytoskeleton Protein EB3 Contributes to Dendritic Spines Enlargement and Enhances Their Resilience to Toxic Effects of Beta-Amyloid
- PMID: 35216391
- PMCID: PMC8875759
- DOI: 10.3390/ijms23042274
Cytoskeleton Protein EB3 Contributes to Dendritic Spines Enlargement and Enhances Their Resilience to Toxic Effects of Beta-Amyloid
Abstract
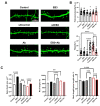
EB3 protein is expressed abundantly in the nervous system and transiently enters the dendritic spines at the tip of the growing microtubule, which leads to spine enlargement. Nevertheless, the role of dynamic microtubules, and particularly EB3 protein, in synapse function is still elusive. By manipulating the EB3 expression level, we have shown that this protein is required for a normal dendritogenesis. Nonetheless, EB3 overexpression also reduces hippocampal neurons dendritic branching and total dendritic length. This effect likely occurs due to the speeding neuronal development cycle from dendrite outgrowth to the step when dendritic spines are forming. Implementing direct morphometric characterization of dendritic spines, we showed that EB3 overexpression leads to a dramatic increase in the dendritic spine head area. EB3 knockout oppositely reduces spine head area and increases spine neck length and spine neck/spine length ratio. The same effect is observed in conditions of amyloid-beta toxicity, modeling Alzheimer`s disease. Neck elongation is supposed to be a common detrimental effect on the spine's shape, which makes them biochemically and electrically less connected to the dendrite. EB3 also potentiates the formation of presynaptic protein Synapsin clusters and CaMKII-alpha preferential localization in spines rather than in dendrites of hippocampal neurons, while its downregulation has an opposite effect and reduces the size of presynaptic protein clusters Synapsin and PSD95. EB3's role in spine development and maturation determines its neuroprotective effect. EB3 overexpression makes dendritic spines resilient to amyloid-beta toxicity, restores altered PSD95 clustering, and reduces CaMKII-alpha localization in spines observed in this pathological state.
Keywords: Alzheimer’s disease; EB3; PSD95; Synapsin CaMKII; beta-amyloid; dendritic spines; end-binding protein; neuronal morphology; neuroprotection.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

