Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules-Effect on Structural and Mechanical Properties
- PMID: 35216412
- PMCID: PMC8877509
- DOI: 10.3390/ijms23042297
Impact of Collagen Crosslinking on Dislocated Human Shoulder Capsules-Effect on Structural and Mechanical Properties
Abstract
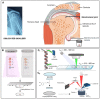
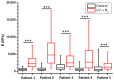
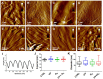
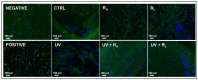
Classical treatments of shoulder instability are associated with recurrence. To determine whether the modification of the capsule properties may be an alternative procedure, the effect of crosslinking treatment on the structure and mechanical properties of diseased human shoulder capsules was investigated. Joint capsules harvested from patients during shoulder surgery (n = 5) were treated or not with UV and/or riboflavin (0.1%, 1.0% and 2.5%). The structure and the mechanical properties of the capsules were determined by atomic force microscopy. The effect of treatments on cell death was investigated. Collagen fibrils were well-aligned and adjacent to each other with a D-periodicity of 66.9 ± 3.2 nm and a diameter of 71.8 ± 15.4 nm in control untreated capsules. No effect of treatments was observed on the organization of the collagen fibrils nor on their intrinsic characteristics, including D-periodicity or their mean diameter. The treatments also did not induce cell death. In contrast, UV + 2.5% riboflavin induced capsule stiffness, as revealed by the increased Young's modulus values (p < 0.0001 for each patient). Our results showed that the crosslinking procedure changed the biomechanics of diseased capsules, while keeping their structural organisation unchanged at the single fibril level. The UV/riboflavin crosslinking procedure may be a promising way to preserve the functions of collagen-based tissues and tune their elasticity for clinically relevant treatments.
Keywords: atomic force microscopy; shoulder instability; type I collagen fibrils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Thomazeau H., Langlais T., Hardy A., Curado J., Herisson O., Mouton J., Charousset C., Courage O., French Arthroscopy Society. Nourissat G. Long-Term, Prospective, Multicenter Study of Isolated Bankart Repair for a Patient Selection Method Based on the Instability Severity Index Score. Am. J. Sports Med. 2019;47:1057–1061. doi: 10.1177/0363546519833920. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

