Employing CRISPR-Cas9 to Generate CD133 Synthetic Lethal Melanoma Stem Cells
- PMID: 35216449
- PMCID: PMC8877091
- DOI: 10.3390/ijms23042333
Employing CRISPR-Cas9 to Generate CD133 Synthetic Lethal Melanoma Stem Cells
Abstract
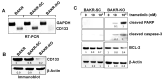
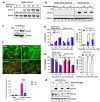
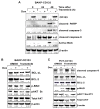
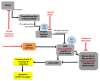
Malignant melanoma is a lethal skin cancer containing melanoma-initiating cells (MIC) implicated in tumorigenesis, invasion, and drug resistance, and is characterized by the elevated expression of stem cell markers, including CD133. The siRNA knockdown of CD133 enhances apoptosis induced by the MEK inhibitor trametinib in melanoma cells. This study investigates the underlying mechanisms of CD133's anti-apoptotic activity in patient-derived BAKP and POT cells, harboring difficult-to-treat NRASQ61K and NRASQ61R drivers, after CRISPR-Cas9 CD133 knockout or Dox-inducible expression of CD133. MACS-sorted CD133(+) BAKP cells were conditionally reprogrammed to derive BAKR cells with sustained CD133 expression and MIC features. Compared to BAKP, CD133(+) BAKR exhibit increased cell survival and reduced apoptosis in response to trametinib or the chemotherapeutic dacarbazine (DTIC). CRISPR-Cas9-mediated CD133 knockout in BAKR cells (BAKR-KO) re-sensitized cells to trametinib. CD133 knockout in BAKP and POT cells increased trametinib-induced apoptosis by reducing anti-apoptotic BCL-xL, p-AKT, and p-BAD and increasing pro-apoptotic BAX. Conversely, Dox-induced CD133 expression diminished apoptosis in both trametinib-treated cell lines, coincident with elevated p-AKT, p-BAD, BCL-2, and BCL-xL and decreased activation of BAX and caspases-3 and -9. AKT1/2 siRNA knockdown or inhibition of BCL-2 family members with navitoclax (ABT-263) in BAKP-KO cells further enhanced caspase-mediated apoptotic PARP cleavage. CD133 may therefore activate a survival pathway where (1) increased AKT phosphorylation and activation induces (2) BAD phosphorylation and inactivation, (3) decreases BAX activation, and (4) reduces caspases-3 and -9 activity and caspase-mediated PARP cleavage, leading to apoptosis suppression and drug resistance in melanoma. Targeting nodes of the CD133, AKT, or BCL-2 survival pathways with trametinib highlights the potential for combination therapies for NRAS-mutant melanoma stem cells for the development of more effective treatments for patients with high-risk melanoma.
Keywords: AKT; CD133; CRISPR/cas9 knockdown; melanoma stem cells; trametinib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cancer Stat Facts: Melanoma of the Skin. Bethesda; Rockville, MD, USA: 2021. [(accessed on 18 May 2021)]. Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/statfacts/html/melan.html.
-
- Paluncic J., Kovacevic Z., Jansson P.J., Kalinowski D., Merlot A.M., Huang M.L.-H., Lok H.C., Sahni S., Lane D.J.R., Richardson D.R. Roads to melanoma: Key pathways and emerging players in melanoma progression and oncogenic signaling. Biochim. Biophys. Acta. 2016;1863:770–784. doi: 10.1016/j.bbamcr.2016.01.025. - DOI - PubMed
-
- Spagnolo F., Picasso V., Lambertini M., Ottaviano V., Dozin B., Queirolo P. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: A systematic review. Cancer Treat. Rev. 2016;45:38–45. doi: 10.1016/j.ctrv.2016.03.003. - DOI - PubMed
-
- Ugurel S., Röhmel J., Ascierto P.A., Flaherty K.T., Grob J.J., Hauschild A., Larkin J., Long G.V., Lorigan P., McArthur G.A., et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur. J. Cancer. 2017;83:247–257. doi: 10.1016/j.ejca.2017.06.028. - DOI - PubMed
-
- Sandri S., Faião-Flores F., Tiago M., Pennacchi P.C., Massaro R.R., Alves-Fernandes D.K., Berardinelli G.N., Evangelista A.F., Vazquez V.D., Reis R.M., et al. Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by MMP-2 upregulation. Pharmacol. Res. 2016;111:523–533. doi: 10.1016/j.phrs.2016.07.017. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

