Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs
- PMID: 35216462
- PMCID: PMC8879019
- DOI: 10.3390/ijms23042345
Ab Initio Insight into the Interaction of Metal-Decorated Fluorinated Carbon Fullerenes with Anti-COVID Drugs
Abstract
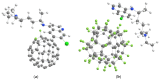
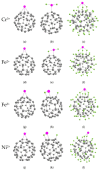
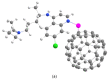
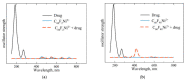
We theoretically investigated the adsorption of two common anti-COVID drugs, favipiravir and chloroquine, on fluorinated C60 fullerene, decorated with metal ions Cr3+, Fe2+, Fe3+, Ni2+. We focused on the effect of fluoridation on the interaction of fullerene with metal ions and drugs in an aqueous solution. We considered three model systems, C60, C60F2 and C60F48, and represented pristine, low-fluorinated and high-fluorinated fullerenes, respectively. Adsorption energies, deformation of fullerene and drug molecules, frontier molecular orbitals and vibrational spectra were investigated in detail. We found that different drugs and different ions interacted differently with fluorinated fullerenes. Cr3+ and Fe2+ ions lead to the defluorination of low-fluorinated fullerenes. Favipiravir also leads to their defluorination with the formation of HF molecules. Therefore, fluorinated fullerenes are not suitable for the delivery of favipiravir and similar drugs molecules. In contrast, we found that fluorine enhances the adsorption of Ni2+ and Fe3+ ions on fullerene and their activity to chloroquine. Ni2+-decorated fluorinated fullerenes were found to be stable and suitable carriers for the loading of chloroquine. Clear shifts of infrared, ultraviolet and visible spectra can provide control over the loading of chloroquine on Ni2+-doped fluorinated fullerenes.
Keywords: COVID-19; chloroquine; density functional theory; drug delivery; favipiravir; fullerenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
DFT calculations towards the geometry optimization, electronic structure, infrared spectroscopy and UV-vis analyses of Favipiravir adsorption on the first-row transition metals doped fullerenes; a new strategy for COVID-19 therapy.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Feb 15;247:119082. doi: 10.1016/j.saa.2020.119082. Epub 2020 Oct 17. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33120121 Free PMC article.
-
Metal oxide nanocage as drug delivery systems for Favipiravir, as an effective drug for the treatment of COVID-19: a computational study.J Mol Model. 2022 Feb 18;28(3):64. doi: 10.1007/s00894-022-05054-6. J Mol Model. 2022. PMID: 35182223 Free PMC article.
-
Fullerene C60 containing porphyrin-like metal center as drug delivery system for ibuprofen drug.J Mol Model. 2019 Dec 13;26(1):7. doi: 10.1007/s00894-019-4267-1. J Mol Model. 2019. PMID: 31834504
-
C60-fullerenes as Drug Delivery Carriers for Anticancer Agents: Promises and Hurdles.Pharm Nanotechnol. 2017;5(3):169-179. doi: 10.2174/2211738505666170301142232. Pharm Nanotechnol. 2017. PMID: 29361902 Review.
-
[Fullerenes in biology].Postepy Biochem. 2007;53(1):91-6. Postepy Biochem. 2007. PMID: 17718393 Review. Polish.
Cited by
-
Nanotechnology in Targeted Drug Delivery.Int J Mol Sci. 2023 May 3;24(9):8194. doi: 10.3390/ijms24098194. Int J Mol Sci. 2023. PMID: 37175903 Free PMC article.
References
-
- Veclani D., Tolazzi M., Melchior A. Molecular Interpretation of Pharmaceuticals’ Adsorption on Carbon Nanomaterials: Theory Meets Experiments. Processes. 2020;8:642. doi: 10.3390/pr8060642. - DOI
-
- Kroto H.W., Heath J.R., O’Brien S.C., Curl R.F., Smalley R.E. C60: Buckminsterfullerene. Nature. 1985;318:162. doi: 10.1038/318162a0. - DOI
-
- Goodarzi S., Da Ros T., Conde J., Sefat F., Mozafari M. Fullerene: Biomedical Engineers Get to Revisit an Old Friend. Materials Today. 2017;20:460. doi: 10.1016/j.mattod.2017.03.017. - DOI
-
- Wang Z., Ke X., Zhu Z., Zhu F., Ruan M., Chen H., Huang R., Zheng L. A New Carbon Solid Made of the World’s Smallest Caged Fullerene C20. Phys. Lett. A. 2001;280:351–356. doi: 10.1016/S0375-9601(00)00847-1. - DOI
-
- Katin K.P., Podlivaev A.I. Dynamic Characteristics of the Low-Temperature Decomposition of the C20 Fullerene. Phys. Solid State. 2010;52:436. doi: 10.1134/S1063783410020356. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

