Thymic stromal lymphopoietin controls hair growth
- PMID: 35216683
- PMCID: PMC9039851
- DOI: 10.1016/j.stemcr.2022.01.017
Thymic stromal lymphopoietin controls hair growth
Abstract
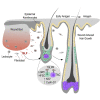
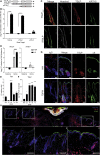
Skin tissue regeneration after injury involves the production and integration of signals by stem cells residing in hair follicles (HFSCs). Much remains unknown about how specific wound-derived factors modulate stem cell contribution to hair growth. We demonstrate that thymic stromal lymphopoietin (TSLP) is produced in response to skin injury and during the anagen phase of the hair cycle. Intradermal injection of TSLP promoted wound-induced hair growth (WIHG), whereas neutralizing TSLP receptor (TSLPR) inhibited WIHG. Using flow cytometry and fluorescent immunostaining, we found that TSLP promoted proliferation of transit-amplifying cells. Lgr5CreER-mediated deletion of Tslpr in HFSCs inhibited both wound-induced and exogenous TSLP-induced hair growth. Our data highlight a novel function for TSLP in regulation of hair follicle activity during homeostasis and wound healing.
Keywords: TSLP; hair follicle; hair follicle stem cell; human; immunology; mouse; regeneration; tissue stem cell.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Abbasi S., Biernaskie J. Injury modifies the fate of hair follicle dermal stem cell progeny in a hair cycle-dependent manner. Exp. Dermatol. 2019;28:419–424. - PubMed
-
- Abbasi S., Sinha S., Labit E., Rosin N.L., Yoon G., Rahmani W., Jaffer A., Sharma N., Hagner A., Shah P., et al. Distinct regulatory programs control the latent regenerative potential of dermal fibroblasts during wound healing. Cell Stem Cell. 2020;27:396–412.e6. - PubMed
-
- Allakhverdi Z., Comeau M.R., Jessup H.K., Yoon B.-R.P., Brewer A., Chartier S., Paquette N., Ziegler S.F., Sarfati M., Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007;204:253–258. - PMC - PubMed
-
- Barker N., Bartfeld S., Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

