Novel Imaging Methods for Renal Mass Characterization: A Collaborative Review
- PMID: 35216855
- PMCID: PMC9844544
- DOI: 10.1016/j.eururo.2022.01.040
Novel Imaging Methods for Renal Mass Characterization: A Collaborative Review
Abstract
Context: The incidental detection of localized renal masses has been rising steadily, but a significant proportion of these tumors are benign or indolent and, in most cases, do not require treatment. At the present time, a majority of patients with an incidentally detected renal tumor undergo treatment for the presumption of cancer, leading to a significant number of unnecessary surgical interventions that can result in complications including loss of renal function. Thus, there exists a clinical need for improved tools to aid in the pretreatment characterization of renal tumors to inform patient management.
Objective: To systematically review the evidence on noninvasive, imaging-based tools for solid renal mass characterization.
Evidence acquisition: The MEDLINE database was systematically searched for relevant studies on novel imaging techniques and interpretative tools for the characterization of solid renal masses, published in the past 10 yr.
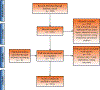
Evidence synthesis: Over the past decade, several novel imaging tools have offered promise for the improved characterization of indeterminate renal masses. Technologies of particular note include multiparametric magnetic resonance imaging of the kidney, molecular imaging with targeted radiopharmaceutical agents, and use of radiomics as well as artificial intelligence to enhance the interpretation of imaging studies. Among these, 99mTc-sestamibi single photon emission computed tomography/computed tomography (CT) for the identification of benign renal oncocytomas and hybrid oncocytic chromophobe tumors, and positron emission tomography/CT imaging with radiolabeled girentuximab for the identification of clear cell renal cell carcinoma, are likely to be closest to implementation in clinical practice.
Conclusions: A number of novel imaging tools stand poised to aid in the noninvasive characterization of indeterminate renal masses. In the future, these tools may aid in patient management by providing a comprehensive virtual biopsy, complete with information on tumor histology, underlying molecular abnormalities, and ultimately disease prognosis.
Patient summary: Not all renal tumors require treatment, as a significant proportion are either benign or have limited metastatic potential. Several innovative imaging tools have shown promise for their ability to improve the characterization of renal tumors and provide guidance in terms of patient management.
Keywords: (99m)Tc-sestamibi; Artificial intelligence; Girentuximab; Kidney cancer; Machine learning; Multiparametric magnetic resonance imaging; PET; Radiomics; Renal cell carcinoma; SPECT; Virtual biopsy.
Copyright © 2022 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


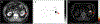
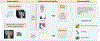
Comment in
-
Too Hot to Handle, Too Cold to Care: The Future of Renal Mass Imaging.Eur Urol. 2022 May;81(5):489-491. doi: 10.1016/j.eururo.2022.02.012. Epub 2022 Mar 8. Eur Urol. 2022. PMID: 35277289 No abstract available.
References
-
- Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–30. - PubMed
-
- Sevcenco S, Heinz-Peer G, Ponhold L, et al. Utility and limitations of 3-Tesla diffusion-weighted magnetic resonance imaging for differentiation of renal tumors. Eur J Radiol 2014;83:909–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

