Diagnostic accuracy of the Abbott ID NOW SARS-CoV-2 rapid test for the triage of acute medical admissions
- PMID: 35217130
- PMCID: PMC8863956
- DOI: 10.1016/j.jhin.2022.02.010
Diagnostic accuracy of the Abbott ID NOW SARS-CoV-2 rapid test for the triage of acute medical admissions
Abstract
Background: Decisions to isolate patients at risk of having coronavirus disease 2019 (COVID-19) in the emergency department (ED) must be rapid and accurate to ensure prompt treatment and maintain patient flow whilst minimising nosocomial spread. Reverse transcription polymerase chain reaction (RT-PCR) assays are too slow to achieve this, and near-patient testing is being used increasingly to facilitate triage. The ID NOW severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) assay is an isothermal nucleic acid amplification near-patient test which targets the RNA-dependent RNA-polymerase gene.
Aim: To assess the diagnostic performance of ID NOW as a COVID-19 triage tool for medical admissions from the ED of a large acute hospital.
Methods: All adult acute medical admissions from the ED between 31st March and 31st July 2021 with valid ID NOW and RT-PCR results were included. The diagnostic accuracy of ID NOW [sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV)] was calculated against the laboratory reference standard. Discrepant results were explored further using cycle threshold values and clinical data.
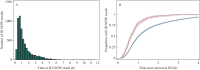
Findings: Two percent (124/6050) of medical admissions were SARS-CoV-2 positive on RT-PCR. Compared with PCR, ID NOW had sensitivity and specificity of 83.1% [95% confidence interval (CI) 75.4-88.7] and 99.5% (95% CI 99.3-99.6), respectively. PPV and NPV were 76.9% (95% CI 69.0-83.2) and 99.6% (95% CI 99.5-99.8), respectively. The median time from arrival in the ED to ID NOW result was 59 min.
Conclusion: ID NOW provides a rapid and reliable adjunct for the safe triage of patients with COVID-19, and can work effectively when integrated into an ED triage algorithm.
Keywords: COVID-19; Emergency department; ID NOW; Near-patient testing; SARS-CoV-2; Triage.
Copyright © 2022 The Healthcare Infection Society. Published by Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The diagnostic accuracy of the ID NOW COVID-19 point of care test in acute hospital admissions.J Clin Virol. 2024 Feb;170:105634. doi: 10.1016/j.jcv.2023.105634. Epub 2023 Dec 13. J Clin Virol. 2024. PMID: 38211537
-
Clinical performance of Abbott ID NOW™ COVID-19 2.0 rapid molecular point-of-care test compared to three real-time RT-PCR assays.Microbiol Spectr. 2025 Mar 4;13(3):e0203324. doi: 10.1128/spectrum.02033-24. Epub 2025 Feb 11. Microbiol Spectr. 2025. PMID: 39932292 Free PMC article.
-
Diagnostic accuracy of the Cepheid Xpert Xpress and the Abbott ID NOW assay for rapid detection of SARS-CoV-2: A systematic review and meta-analysis.J Med Virol. 2021 Jul;93(7):4523-4531. doi: 10.1002/jmv.26994. Epub 2021 May 3. J Med Virol. 2021. PMID: 33913533 Free PMC article.
-
Evaluating the Ability to ID (COVID-19) NOW: a Large Real-World Prospective Evaluation of the Abbott ID NOW COVID-19 Assay.Microbiol Spectr. 2022 Jun 29;10(3):e0051322. doi: 10.1128/spectrum.00513-22. Epub 2022 May 17. Microbiol Spectr. 2022. PMID: 35579469 Free PMC article.
-
Sensitivity of ID NOW and RT-PCR for detection of SARS-CoV-2 in an ambulatory population.Elife. 2021 Apr 20;10:e65726. doi: 10.7554/eLife.65726. Elife. 2021. PMID: 33876726 Free PMC article.
Cited by
-
Evaluation of a Commercial Rapid Molecular Point-of-Care Assay for Differential Diagnosis Between SARS-CoV-2 and Flu A/B Infections in a Pediatric Setting.Viruses. 2024 Oct 20;16(10):1638. doi: 10.3390/v16101638. Viruses. 2024. PMID: 39459970 Free PMC article.
-
[Implementation of isotermic PCR for detection of SARS-CoV-2 virus].Rev Med Inst Mex Seguro Soc. 2024 May 6;62(3):1-6. doi: 10.5281/zenodo.10998931. Rev Med Inst Mex Seguro Soc. 2024. PMID: 39530769 Free PMC article. Spanish.
-
Point-of-Care Testing for SARS-CoV-2: A Prospective Study in a Primary Health Centre.Diagnostics (Basel). 2023 May 28;13(11):1888. doi: 10.3390/diagnostics13111888. Diagnostics (Basel). 2023. PMID: 37296741 Free PMC article.
-
The COVID-19 pandemic and critical laboratory functions. Can fast-track molecular testing reduce work absence in the laboratory?J Infect Prev. 2025 Apr 3:17571774251330455. doi: 10.1177/17571774251330455. Online ahead of print. J Infect Prev. 2025. PMID: 40190997 Free PMC article.
-
Does the Addition of Point-of-Care Testing Alter Antibiotic Prescribing Decisions When Patients Present with Acute Sore Throat to Primary Care? A Prospective Test of Change.Diagnostics (Basel). 2024 May 26;14(11):1104. doi: 10.3390/diagnostics14111104. Diagnostics (Basel). 2024. PMID: 38893631 Free PMC article.
References
-
- UK Government . UK Government; London: 2022. Healthcare in United Kingdom.https://coronavirus.data.gov.uk/details/healthcare Available at: [last accessed January 2022]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

