Triaxial detection of the neuromagnetic field using optically-pumped magnetometry: feasibility and application in children
- PMID: 35217205
- PMCID: PMC9135302
- DOI: 10.1016/j.neuroimage.2022.119027
Triaxial detection of the neuromagnetic field using optically-pumped magnetometry: feasibility and application in children
Abstract
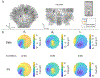
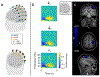
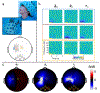
Optically-pumped magnetometers (OPMs) are an established alternative to superconducting sensors for magnetoencephalography (MEG), offering significant advantages including flexibility to accommodate any head size, uniform coverage, free movement during scanning, better data quality and lower cost. However, OPM sensor technology remains under development; there is flexibility regarding OPM design and it is not yet clear which variant will prove most effective for MEG. Most OPM-MEG implementations have either used single-axis (equivalent to conventional MEG) or dual-axis magnetic field measurements. Here we demonstrate use of a triaxial OPM formulation, able to characterise the full 3D neuromagnetic field vector. We show that this novel sensor is able to characterise magnetic fields with high accuracy and sensitivity that matches conventional (dual-axis) OPMs. We show practicality via measurement of biomagnetic fields from both the heart and the brain. Using simulations, we demonstrate how triaxial measurement offers improved cortical coverage, especially in infants. Finally, we introduce a new 3D-printed child-friendly OPM-helmet and demonstrate feasibility of triaxial measurement in a five-year-old. In sum, the data presented demonstrate that triaxial OPMs offer a significant improvement over dual-axis variants and are likely to become the sensor of choice for future MEG systems, particularly for deployment in paediatric populations.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest V.S. is the founding director of QuSpin, the commercial entity selling OPM magnetometers. J.O. and C.D. are employees of QuSpin. E.B. and M.J.B. are directors of Cerca Magnetics Limited, a spin-out company whose aim is to commercialise aspects of OPM-MEG technology. E.B., M.J.B., R.B., N.H. and R.M.H. hold founding equity in Cerca Magnetics Limited.
Figures

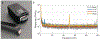



References
-
- Bloch F, 1946. Nuclear Induction. Phys. Rev 70, 460.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

