Modeling suggests gene editing combined with vaccination could eliminate a persistent disease in livestock
- PMID: 35217603
- PMCID: PMC8892294
- DOI: 10.1073/pnas.2107224119
Modeling suggests gene editing combined with vaccination could eliminate a persistent disease in livestock
Abstract
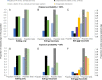
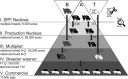
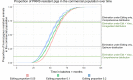
Recent breakthroughs in gene-editing technologies that can render individual animals fully resistant to infections may offer unprecedented opportunities for controlling future epidemics in farm animals. Yet, their potential for reducing disease spread is poorly understood as the necessary theoretical framework for estimating epidemiological effects arising from gene-editing applications is currently lacking. Here, we develop semistochastic modeling approaches to investigate how the adoption of gene editing may affect infectious disease prevalence in farmed animal populations and the prospects and time scale for disease elimination. We apply our models to the porcine reproductive and respiratory syndrome (PRRS), one of the most persistent global livestock diseases to date. Whereas extensive control efforts have shown limited success, recent production of gene-edited pigs that are fully resistant to the PRRS virus have raised expectations for eliminating this deadly disease. Our models predict that disease elimination on a national scale would be difficult to achieve if gene editing was used as the only disease control. However, from a purely epidemiological perspective, disease elimination may be achievable within 3 to 6 y, if gene editing were complemented with widespread and sufficiently effective vaccination. Besides strategic distribution of genetically resistant animals, several other key determinants underpinning the epidemiological impact of gene editing were identified.
Keywords: CRISPR/Cas9; PRRS; gene editing; infectious disease; mathematical model.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: Although the University of Edinburgh’s Roslin Institute and the animal genetics company Genus plc have signed an agreement to produce pigs that are resistant to a respiratory disease affecting livestock worldwide (https://www.ed.ac.uk/roslin/news-events/latest-news/agreement-targets-disease-resistant-gene-edited-pi), this study, carried out prior to the agreement, builds solely on published findings and rigorous scientific methods for model development and assessment. As such the results are entirely objective, and neither the results nor their interpretation are in any way influenced by this agreement or by personal beliefs or self-interest.
Figures


References
-
- Dubock A., Golden rice: Instructions for use. Agric. Food Secur. 6, 60 (2017).
-
- World Food Programme, 2019 - Hunger map. https://www.wfp.org/publications/2019-hunger-map. Accessed 13 August 2019.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

